Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells
- PMID: 24040145
- PMCID: PMC3769353
- DOI: 10.1371/journal.pone.0074010
Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells
Abstract
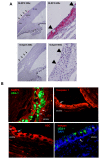
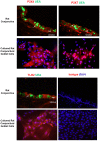
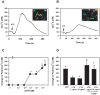
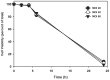
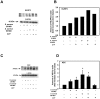
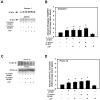
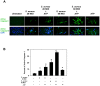
The conjunctiva is a moist mucosal membrane that is constantly exposed to an array of potential pathogens and triggers of inflammation. The NACHT, leucine rich repeat (LRR), and pyrin domain-containing protein 3 (NLRP3) is a Nod-like receptor that can sense pathogens or other triggers, and is highly expressed in wet mucosal membranes. NLRP3 is a member of the multi-protein complex termed the NLRP3 inflammasome that activates the caspase 1 pathway, inducing the secretion of biologically active IL-1β, a major initiator and promoter of inflammation. The purpose of this study was to: (1) determine whether NLRP3 is expressed in the conjunctiva and (2) determine whether goblet cells specifically contribute to innate mediated inflammation via secretion of IL-1β. We report that the receptors known to be involved in the priming and activation of the NLRP3 inflammasome, the purinergic receptors P2X4 and P2X7 and the bacterial Toll-like receptor 2 are present and functional in conjunctival goblet cells. Toxin-containing Staphylococcus aureus (S. aureus), which activates the NLRP3 inflammasome, increased the expression of the inflammasome proteins NLRP3, ASC and pro- and mature caspase 1 in conjunctival goblet cells. The biologically active form of IL-1β was detected in goblet cell culture supernatants in response to S. aureus, which was reduced when the cells were treated with the caspase 1 inhibitor Z-YVAD. We conclude that the NLRP3 inflammasome components are present in conjunctival goblet cells. The NRLP3 inflammasome appears to be activated in conjunctival goblet cells by toxin-containing S. aureus via the caspase 1 pathway to secrete mature IL1-β. Thus goblet cells contribute to the innate immune response in the conjunctiva by activation of the NLRP3 inflammasome.
Conflict of interest statement
Figures



References
-
- Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10: 417–426. - PubMed
-
- Anders HJ, Muruve DA (2011) The inflammasomes in kidney disease. J Am Soc Nephrol 22: 1007–1018. - PubMed
-
- Mariathasan S (2007) ASC, Ipaf and Cryopyrin/Nalp3: bona fide intracellular adapters of the caspase-1 inflammasome. Microbes Infect 9: 664–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

