Non-catalytic site HIV-1 integrase inhibitors disrupt core maturation and induce a reverse transcription block in target cells
- PMID: 24040198
- PMCID: PMC3767657
- DOI: 10.1371/journal.pone.0074163
Non-catalytic site HIV-1 integrase inhibitors disrupt core maturation and induce a reverse transcription block in target cells
Abstract
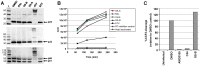
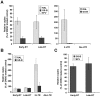
HIV-1 integrase (IN) is the target for two classes of antiretrovirals: i) the integrase strand-transfer inhibitors (INSTIs) and ii) the non-catalytic site integrase inhibitors (NCINIs). NCINIs bind at the IN dimer interface and are thought to interfere primarily with viral DNA (vDNA) integration in the target cell by blocking IN-vDNA assembly as well as the IN-LEDGF/p75 interaction. Herein we show that treatment of virus-producing cells, but not of mature virions or target cells, drives NCINI antiviral potency. NCINIs target an essential late-stage event in HIV replication that is insensitive to LEDGF levels in the producer cells. Virus particles produced in the presence of NCINIs displayed normal Gag-Pol processing and endogenous reverse transcriptase activity, but were defective at initiating vDNA synthesis following entry into the target cell. NCINI-resistant virus carrying a T174I mutation in the IN dimer interface was less sensitive to the compound-induced late-stage effects, including the reverse transcription block. Wild-type, but not T174I virus, produced in the presence of NCINIs exhibited striking defects in core morphology and an increased level of IN oligomers that was not observed upon treatment of mature cell-free particles. Collectively, these results reveal that NCINIs act through a novel mechanism that is unrelated to the previously observed inhibition of IN activity or IN-LEDGF interaction, and instead involves the disruption of an IN function during HIV-1 core maturation and assembly.
Conflict of interest statement
Figures





References
-
- Pommier Y, Johnson AA, Marchand C (2005) Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov 4: 236–248. - PubMed
-
- Al-Mawsawi LQ, Neamati N (2007) Blocking interactions between HIV-1 integrase and cellular cofactors: an emerging anti-retroviral strategy. Trends Pharmacol Sci 28: 526–535. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

