Prion protein accumulation in lipid rafts of mouse aging brain
- PMID: 24040215
- PMCID: PMC3769255
- DOI: 10.1371/journal.pone.0074244
Prion protein accumulation in lipid rafts of mouse aging brain
Abstract
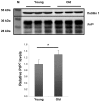
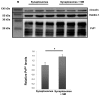
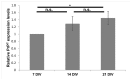
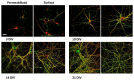
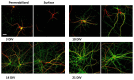
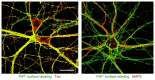
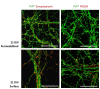
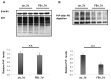
The cellular form of the prion protein (PrP(C)) is a normal constituent of neuronal cell membranes. The protein misfolding causes rare neurodegenerative disorders known as transmissible spongiform encephalopathies or prion diseases. These maladies can be sporadic, genetic or infectious. Sporadic prion diseases are the most common form mainly affecting aging people. In this work, we investigate the biochemical environment in which sporadic prion diseases may develop, focusing our attention on the cell membrane of neurons in the aging brain. It is well established that with aging the ratio between the most abundant lipid components of rafts undergoes a major change: while cholesterol decreases, sphingomyelin content rises. Our results indicate that the aging process modifies the compartmentalization of PrP(C). In old mice, this change favors PrP(C) accumulation in detergent-resistant membranes, particularly in hippocampi. To confirm the relationship between lipid content changes and PrP(C) translocation into detergent-resistant membranes (DRMs), we looked at PrP(C) compartmentalization in hippocampi from acid sphingomyelinase (ASM) knockout (KO) mice and synaptosomes enriched in sphingomyelin. In the presence of high sphingomyelin content, we observed a significant increase of PrP(C) in DRMS. This process is not due to higher levels of total protein and it could, in turn, favor the onset of sporadic prion diseases during aging as it increases the PrP intermolecular contacts into lipid rafts. We observed that lowering sphingomyelin in scrapie-infected cells by using fumonisin B1 led to a 50% decrease in protease-resistant PrP formation. This may suggest an involvement of PrP lipid environment in prion formation and consequently it may play a role in the onset or development of sporadic forms of prion diseases.
Conflict of interest statement
Figures


References
-
- Ford MJ, Burton LJ, Li H, Graham CH, Frobert Y et al. (2002) A marked disparity between the expression of prion protein and its message by neurones of the CNS. Neuroscience 111: 533-551. doi:10.1016/S0306-4522(01)00603-0. PubMed: 12031342. - DOI - PubMed
-
- Salès N, Hässig R, Rodolfo K, Di Giamberardino L, Traiffort E et al. (2002) Developmental expression of the cellular prion protein in elongating axons. Eur J Neurosci 15: 1163-1177. doi:10.1046/j.1460-9568.2002.01953.x. PubMed: 11982627. - DOI - PubMed
-
- Benvegnù S, Poggiolini I, Legname G (2010) Neurodevelopmental expression and localization of the cellular prion protein in the central nervous system of the mouse. J Comp Neurol 518: 1879-1891. doi:10.1002/cne.22357. PubMed: 20394048. - DOI - PubMed
-
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A et al. (1993) Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A 90: 10962-10966. doi:10.1073/pnas.90.23.10962. PubMed: 7902575. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

