Rapid antemortem detection of CWD prions in deer saliva
- PMID: 24040235
- PMCID: PMC3770611
- DOI: 10.1371/journal.pone.0074377
Rapid antemortem detection of CWD prions in deer saliva
Abstract
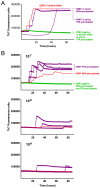
Chronic wasting disease (CWD) is an efficiently transmitted prion disease of cervids, now identified in 22 United States, 2 Canadian provinces and Korea. One hallmark of CWD is the shedding of infectious prions in saliva, as demonstrated by bioassay in deer. It is also clear that the concentration of prions in saliva, blood, urine and feces is much lower than in the nervous system or lymphoid tissues. Rapid in vitro detection of CWD (and other) prions in body fluids and excreta has been problematic due to the sensitivity limits of direct assays (western blotting, ELISA) and the presence of inhibitors in these complex biological materials that hamper detection. Here we use real-time quaking induced conversion (RT-QuIC) to demonstrate CWD prions in both diluted and prion-enriched saliva samples from asymptomatic and symptomatic white-tailed deer. CWD prions were detected in 14 of 24 (58.3%) diluted saliva samples from CWD-exposed white-tailed deer, including 9 of 14 asymptomatic animals (64.2%). In addition, a phosphotungstic acid enrichment enhanced the RT-QuIC assay sensitivity, enabling detection in 19 of 24 (79.1%) of the above saliva samples. Bioassay in Tg[CerPrP] mice confirmed the presence of infectious prions in 2 of 2 RT-QuIC-positive saliva samples so examined. The modified RT-QuIC analysis described represents a non-invasive, rapid ante-mortem detection of prions in complex biologic fluids, excreta, or environmental samples as well as a tool for exploring prion trafficking, peripheralization, and dissemination.
Conflict of interest statement
Figures



References
-
- Heisey DM, Mickelsen NA, Schneider JR, Johnson CJ, Johnson CJ et al. (2010) Chronic wasting disease (CWD) susceptibility of several North American rodents that are sympatric with cervid CWD epidemics. J Virol 84: 210-215. doi:10.1128/JVI.00560-09. PubMed: 19828611. - DOI - PMC - PubMed
-
- Sigurdson CJ, Mathiason CK, Perrott MR, Eliason GA, Spraker TR et al. (2008) Experimental chronic wasting disease (CWD) in the ferret. J Comp Pathol 138: 189-196. doi:10.1016/j.jcpa.2008.01.004. PubMed: 18387626. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

