Stepwise assembly of fibrinogen is assisted by the endoplasmic reticulum lectin-chaperone system in HepG2 cells
- PMID: 24040290
- PMCID: PMC3769264
- DOI: 10.1371/journal.pone.0074580
Stepwise assembly of fibrinogen is assisted by the endoplasmic reticulum lectin-chaperone system in HepG2 cells
Abstract
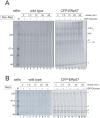
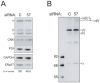
The endoplasmic reticulum (ER) plays essential roles in protein folding and assembly of secretory proteins. ER-resident molecular chaperones and related enzymes assist in protein maturation by co-operated interactions and modifications. However, the folding/assembly of multimeric proteins is not well understood. Here, we show that the maturation of fibrinogen, a hexameric secretory protein (two trimers from α, β and γ subunits), occurs in a stepwise manner. The αγ complex, a precursor for the trimer, is retained in the ER by lectin-like chaperones, and the β subunit is incorporated into the αγ complex immediately after translation. ERp57, a protein disulfide isomerase homologue, is involved in the hexamer formation from two trimers. Our results indicate that the fibrinogen hexamer is formed sequentially, rather than simultaneously, using kinetic pause by lectin chaperones. This study provides a novel insight into the assembly of most abundant multi-subunit secretory proteins.
Conflict of interest statement
Figures




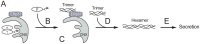
References
-
- Eriksson KK (2004) EDEM Contributes to Maintenance of Protein Folding Efficiency and Secretory Capacity. Journal of Biological Chemistry 279: 44600–44605 doi:10.1074/jbc.M407972200 - DOI - PubMed
-
- Braakman I, Bulleid NJ (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem 80: 71–99 doi:10.1146/annurev.biochem.69.1.69 - DOI - PubMed
-
- Trombetta ES (2003) The contribution of N-glycans and their processing in the endoplasmic reticulum to glycoprotein biosynthesis. Glycobiology 13: 77R–91 doi:10.1093/glycob/cwg075 - DOI - PubMed
-
- Schrag JD, Procopio DO, Cygler M, Thomas DY, Bergeron JJM (2003) Lectin control of protein folding and sorting in the secretory pathway. Trends Biochem Sci 28: 49–57. - PubMed
-
- Ellgaard L, Molinari M, Helenius A (1999) Setting the standards: quality control in the secretory pathway. Science 286: 1882–1888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

