Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease
- PMID: 24040360
- PMCID: PMC3769300
- DOI: 10.1371/journal.pone.0074905
Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease
Abstract
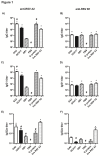
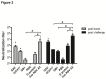
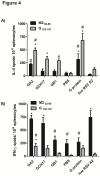
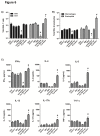
Nanoparticle vaccines were produced using layer-by-layer fabrication and incorporating respiratory syncytial virus (RSV) G protein polypeptides comprising the CX3C chemokine motif. BALB/c mice immunized with G protein nanoparticle vaccines produced a neutralizing antibody response that inhibited RSV replication in the lungs following RSV challenge. ELISPOT analysis showed that G nanoparticle vaccinated mice had increased levels of RSV G protein-specific IL-4 and IFN-γ secreting cells compared to controls following RSV challenge. Remarkably, RSV challenge of G protein nanoparticle vaccinated mice resulted in increased RSV M2-specific IL-4 and IFN-γ secreting T cells, and increased M2-specific H-2Kd-tetramer positive CD8(+) T cells in the lungs compared to controls. Cell type analysis showed vaccination was not associated with increased pulmonary eosinophilia following RSV challenge. These results demonstrate that vaccination of mice with the RSV G protein nanoparticle vaccines induces a potent neutralizing antibody response, increased G protein- and M2-specific T cell responses, and a reduction in RSV disease pathogenesis.
Conflict of interest statement
Figures


Similar articles
-
Virus-Like Particle Vaccine Containing the F Protein of Respiratory Syncytial Virus Confers Protection without Pulmonary Disease by Modulating Specific Subsets of Dendritic Cells and Effector T Cells.J Virol. 2015 Nov;89(22):11692-705. doi: 10.1128/JVI.02018-15. Epub 2015 Sep 9. J Virol. 2015. PMID: 26355098 Free PMC article.
-
Mutating the CX3C Motif in the G Protein Should Make a Live Respiratory Syncytial Virus Vaccine Safer and More Effective.J Virol. 2017 Apr 28;91(10):e02059-16. doi: 10.1128/JVI.02059-16. Print 2017 May 15. J Virol. 2017. PMID: 28275196 Free PMC article.
-
A novel respiratory syncytial virus (RSV) F subunit vaccine adjuvanted with GLA-SE elicits robust protective TH1-type humoral and cellular immunity in rodent models.PLoS One. 2015 Mar 20;10(3):e0119509. doi: 10.1371/journal.pone.0119509. eCollection 2015. PLoS One. 2015. PMID: 25793508 Free PMC article.
-
Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease.Immunol Res. 2007;39(1-3):225-39. doi: 10.1007/s12026-007-0071-6. Immunol Res. 2007. PMID: 17917067 Review.
-
The role of respiratory syncytial virus G protein in immune cell infection and pathogenesis.EBioMedicine. 2024 Sep;107:105318. doi: 10.1016/j.ebiom.2024.105318. Epub 2024 Aug 31. EBioMedicine. 2024. PMID: 39217853 Free PMC article. Review.
Cited by
-
Respiratory Syncytial Virus (RSV) G Protein Vaccines With Central Conserved Domain Mutations Induce CX3C-CX3CR1 Blocking Antibodies.Viruses. 2021 Feb 23;13(2):352. doi: 10.3390/v13020352. Viruses. 2021. PMID: 33672319 Free PMC article.
-
Microparticle RSV Vaccines Presenting the G Protein CX3C Chemokine Motif in the Context of TLR Signaling Induce Protective Th1 Immune Responses and Prevent Pulmonary Eosinophilia Post-Challenge.Vaccines (Basel). 2022 Dec 5;10(12):2078. doi: 10.3390/vaccines10122078. Vaccines (Basel). 2022. PMID: 36560488 Free PMC article.
-
Utility of the Neonatal Calf Model for Testing Vaccines and Intervention Strategies for Use against Human RSV Infection.Vaccines (Basel). 2019 Jan 8;7(1):7. doi: 10.3390/vaccines7010007. Vaccines (Basel). 2019. PMID: 30626099 Free PMC article. Review.
-
Intranasal Vaccination with a Respiratory-Syncytial-Virus-Based Virus-like Particle Displaying the G Protein Conserved Region Induces Severe Weight Loss and Pathology upon Challenge with Wildtype Respiratory Syncytial Virus.Viruses. 2024 May 24;16(6):843. doi: 10.3390/v16060843. Viruses. 2024. PMID: 38932136 Free PMC article.
-
Benzimidazole analogs inhibit respiratory syncytial virus G protein function.Antiviral Res. 2015 Sep;121:31-8. doi: 10.1016/j.antiviral.2015.06.016. Epub 2015 Jun 24. Antiviral Res. 2015. PMID: 26116756 Free PMC article.
References
-
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM et al. (2009) The burden of respiratory syncytial virus infection in young children. N Engl J Med 360: 588-598. doi:10.1056/NEJMoa0804877. PubMed: 19196675. - DOI - PMC - PubMed
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA et al. (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375: 1545-1555. doi:10.1016/S0140-6736(10)60206-1. PubMed: 20399493. - DOI - PMC - PubMed
-
- Dowell SF, Anderson LJ, Gary HE Jr., Erdman DD, Plouffe JF et al. (1996) Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis 174: 456-462. doi:10.1093/infdis/174.3.456. PubMed: 8769600. - DOI - PubMed
-
- Falsey AR (2007) Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med 28: 171-181. doi:10.1055/s-2007-976489. PubMed: 17458771. - DOI - PubMed
-
- Denny FW, Clyde WA Jr., Collier AM, Fernald GW, Henderson FW (1979) The longitudinal approach to the pathogenesis of respiratory disease. Rev Infect Dis 1: 1007-1015. doi:10.1093/clinids/1.6.1007. PubMed: 121783. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

