Engineering yeast hexokinase 2 for improved tolerance toward xylose-induced inactivation
- PMID: 24040384
- PMCID: PMC3765440
- DOI: 10.1371/journal.pone.0075055
Engineering yeast hexokinase 2 for improved tolerance toward xylose-induced inactivation
Abstract
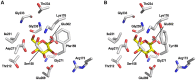
Hexokinase 2 (Hxk2p) from Saccharomyces cerevisiae is a bi-functional enzyme being both a catalyst and an important regulator in the glucose repression signal. In the presence of xylose Hxk2p is irreversibly inactivated through an autophosphorylation mechanism, affecting all functions. Consequently, the regulation of genes involved in sugar transport and fermentative metabolism is impaired. The aim of the study was to obtain new Hxk2p-variants, immune to the autophosphorylation, which potentially can restore the repressive capability closer to its nominal level. In this study we constructed the first condensed, rationally designed combinatorial library targeting the active-site in Hxk2p. We combined protein engineering and genetic engineering for efficient screening and identified a variant with Phe159 changed to tyrosine. This variant had 64% higher catalytic activity in the presence of xylose compared to the wild-type and is expected to be a key component for increasing the productivity of recombinant xylose-fermenting strains for bioethanol production from lignocellulosic feedstocks.
Conflict of interest statement
Figures



References
-
- Lobo Z, Maitra PK (1977) Physiological role of glucose-phosphorylating enzymes in Saccharomyces cerevisiae . Arch Biochem Biophys 182: 639–645. - PubMed
-
- Fernandez R, Herrero P, Gascon S, Moreno F (1984) Xylose-induced decrease in hexokinase PII activity confers resistance to carbon catabolite repression of invertase synthesis in Saccharomyces carlsbergensis . Arch Microbiol 139: 139–142.
-
- Moreno F, Herrero P (2002) The hexokinase 2-dependent glucose signal transduction pathway of Saccharomyces cerevisiae . FEMS Microbiol Rev 26: 83–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

