Fine tuning of spatial arrangement of enzymes in a PCNA-mediated multienzyme complex using a rigid poly-L-proline linker
- PMID: 24040392
- PMCID: PMC3764174
- DOI: 10.1371/journal.pone.0075114
Fine tuning of spatial arrangement of enzymes in a PCNA-mediated multienzyme complex using a rigid poly-L-proline linker
Abstract
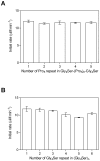
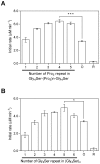
Inspired by natural multienzyme complexes, many types of artificial multienzyme complexes have recently been constructed. We previously constructed a self-assembled complex of a bacterial cytochrome P450 and its ferredoxin and ferredoxin reductase partners using heterotrimerization of proliferating cell nuclear antigen (PCNA) from Sulfolobus solfataricus. In this study, we inserted different peptide linkers between ferredoxin and the PCNA subunit, and examined the effect on activity of the self-assembled multienzyme complex. Although the activity was affected by the lengths of both the rigid poly-L-proline-rich linkers and the flexible Gly4-Ser repeating linkers, the poly-L-proline-rich linkers provided the greatest activity enhancement. The optimized poly-L-proline-rich linker enhanced the activity 1.9-fold compared with the GGGGSLVPRGSGGGGS linker used in the previously reported complex, while the Gly4-Ser repeating linkers, (G4S)n (n = 1-6), did not yield higher activity than the maximum activity by the optimized poly-L-proline linker. Both the rigidity/flexibility and length of the peptide linker were found to be important for enhancing the overall activity of the multienzyme complex.
Conflict of interest statement
Figures





References
-
- Schoffelen S, Van Hest JCM (2012) Multi-enzyme systems: bringing enzymes together in vitro. Soft Matter 8: 1736-1746. doi:10.1039/c1sm06452e. - DOI
-
- Delebecque CJ, Lindner AB, Silver PA, Aldaye FA (2011) Organization of intracellular reactions with rationally designed RNA assemblies. Science 333: 470–474. doi:10.1126/science.1206938. PubMed: 21700839. - DOI - PubMed
-
- Conrado RJ, Wu GC, Boock JT, Xu H, Chen SY et al. (2012) DNA-guided assembly of biosynthetic pathways promotes improved catalytic efficiency. Nucleic Acids Res 40: 1879–1889. doi:10.1093/nar/gkr888. PubMed: 22021385. - DOI - PMC - PubMed
-
- Erkelenz M, Kuo CH, Niemeyer CM (2011) DNA-mediated assembly of cytochrome P450 BM3 subdomains. J Am Chem Soc 133: 16111–16118. doi:10.1021/ja204993s. PubMed: 21919448. - DOI - PubMed
-
- Fu J, Liu M, Liu Y, Woodbury NW, Yan H (2012) Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J Am Chem Soc 134: 5516–5519. doi:10.1021/ja300897h. PubMed: 22414276. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

