18 F-click labeling and preclinical evaluation of a new 18 F-folate for PET imaging
- PMID: 24041035
- PMCID: PMC3849131
- DOI: 10.1186/2191-219X-3-68
18 F-click labeling and preclinical evaluation of a new 18 F-folate for PET imaging
Abstract
Background: The folate receptor (FR) is a well-established target for tumor imaging and therapy. To date, only a few 18 F-folate conjugates via 18 F-prosthetic group labeling for positron emission tomography (PET) imaging have been developed. To some extent, they all lack the optimal balance between efficient radiochemistry and favorable in vivo characteristics.
Methods: A new clickable olate precursor was synthesized by regioselective coupling of folic acid to 11-azido-3,6,9-trioxaundecan-1-amine at the γ-position of the glutamic acid residue. The non-radioactive reference compound was synthesized via copper-catalyzed azide-alkyne cycloaddition of 3-(2-(2-(2-fluoroethoxy)ethoxy)ethoxy)prop-1-yne and γ-(11-azido-3,6,9-trioxaundecanyl)folic acid amide. The radiosynthesis was accomplished in two steps: at first a 18 F-fluorination of 2-(2-(2-(prop-2-yn-1-yloxy)ethoxy)ethoxy)ethyl-4-methylbenzenesulfonate, followed by a 18 F-click reaction with the γ-azido folate. The in vitro, ex vivo, and in vivo behaviors of the new 18 F-folate were investigated using FR-positive human KB cells in displacement assays and microPET studies using KB tumor-bearing mice.
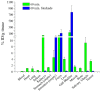
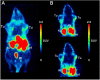
Results: The new 18 F-folate with oligoethylene spacers showed reduced lipophilicity in respect to the previously developed 18 F-click folate with alkyl spacers and excellent affinity (Ki = 1.6 nM) to the FR. Combining the highly efficient 18 F-click chemistry and a polar oligoethylene-based 18 F-prosthetic group facilitated these results. The overall radiochemical yield of the isolated and formulated product averages 8.7%. In vivo PET imaging in KB tumor-bearing mice showed a tumor uptake of 3.4% ID/g tissue, which could be reduced by FR blockade with native folic acid. Although the new 18 F-oligoethyleneglycole (OEG)-folate showed reduced hepatobiliary excretion over time, a distinct unspecific abdominal background was still observed.
Conclusions: A new 18 F-folate was developed, being available in very high radiochemical yields via a fast and convenient two-step radiosynthesis. The new 18 F-OEG-folate showed good in vivo behavior and lines up with several recently evaluated 18 F-labeled folates.
Figures


Similar articles
-
Fluorine-18 click radiosynthesis and preclinical evaluation of a new 18F-labeled folic acid derivative.Bioconjug Chem. 2008 Dec;19(12):2462-70. doi: 10.1021/bc800356r. Bioconjug Chem. 2008. PMID: 19053298
-
[18F]fluoro-deoxy-glucose folate: a novel PET radiotracer with improved in vivo properties for folate receptor targeting.Bioconjug Chem. 2012 Apr 18;23(4):805-13. doi: 10.1021/bc200660z. Epub 2012 Mar 13. Bioconjug Chem. 2012. PMID: 22372827
-
A new 18F-labeled folic acid derivative with improved properties for the PET imaging of folate receptor-positive tumors.J Nucl Med. 2010 Nov;51(11):1756-62. doi: 10.2967/jnumed.110.079756. Epub 2010 Oct 18. J Nucl Med. 2010. PMID: 20956469
-
(18) F-labeled folic acid derivatives for imaging of the folate receptor via positron emission tomography.J Labelled Comp Radiopharm. 2013 Jul-Aug;56(9-10):432-40. doi: 10.1002/jlcr.3104. J Labelled Comp Radiopharm. 2013. PMID: 24285516 Review.
-
3'-Aza-2'-[18F]fluorofolic acid.2013 Apr 6 [updated 2013 Jun 6]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2013 Apr 6 [updated 2013 Jun 6]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23741764 Free Books & Documents. Review.
Cited by
-
Hetero-Diels-Alder and CuAAC Click Reactions for Fluorine-18 Labeling of Peptides: Automation and Comparative Study of the Two Methods.Molecules. 2024 Jul 5;29(13):3198. doi: 10.3390/molecules29133198. Molecules. 2024. PMID: 38999148 Free PMC article.
-
Comparison Study of Two Differently Clicked 18F-Folates-Lipophilicity Plays a Key Role.Pharmaceuticals (Basel). 2018 Mar 17;11(1):30. doi: 10.3390/ph11010030. Pharmaceuticals (Basel). 2018. PMID: 29562610 Free PMC article.
-
18F-labeling using click cycloadditions.Biomed Res Int. 2014;2014:361329. doi: 10.1155/2014/361329. Epub 2014 May 27. Biomed Res Int. 2014. PMID: 25003110 Free PMC article. Review.
-
Development of Folate Receptor-Targeted PET Radiopharmaceuticals for Tumor Imaging-A Bench-to-Bedside Journey.Cancers (Basel). 2020 Jun 9;12(6):1508. doi: 10.3390/cancers12061508. Cancers (Basel). 2020. PMID: 32527010 Free PMC article. Review.
-
Recent Advances in Bioorthogonal Click Chemistry for Efficient Synthesis of Radiotracers and Radiopharmaceuticals.Molecules. 2019 Oct 2;24(19):3567. doi: 10.3390/molecules24193567. Molecules. 2019. PMID: 31581645 Free PMC article. Review.
References
-
- McHugh M. Demonstration of a high affinity membranes and its characterization folate binder in cultured in human cell. J Biol Chem. 1979;3:11312–11318. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

