Bidentate ligands on osmium(VI) nitrido complexes control intracellular targeting and cell death pathways
- PMID: 24041161
- PMCID: PMC3791136
- DOI: 10.1021/ja4075375
Bidentate ligands on osmium(VI) nitrido complexes control intracellular targeting and cell death pathways
Abstract
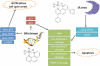
The cellular response evoked by antiproliferating osmium(VI) nitrido compounds of general formula OsN(N^N)Cl3 (N^N = 2,2'-bipyridine 1, 1,10-phenanthroline 2, 3,4,7,8-tetramethyl-1,10-phenanthroline 3, or 4,7-diphenyl-1,10-phenanthroline 4) can be tuned by subtle ligand modifications. Complex 2 induces DNA damage, resulting in activation of the p53 pathway, cell cycle arrest at the G2/M phase, and caspase-dependent apoptotic cell death. In contrast, 4 evokes endoplasmic reticulum (ER) stress leading to the upregulation of proteins of the unfolded protein response pathway, increase in ER size, and p53-independent apoptotic cell death. To the best of our knowledge, 4 is the first osmium compound to induce ER stress in cancer cells.
Figures


Similar articles
-
Synthesis, characterization, and antitumor activity of unusual pseudo five coordinate gold(III) complexes: distinct cytotoxic mechanism or expensive ligand delivery systems?J Inorg Biochem. 2014 Dec;141:121-131. doi: 10.1016/j.jinorgbio.2014.08.014. Epub 2014 Sep 6. J Inorg Biochem. 2014. PMID: 25243390
-
Mixed-ligand ruthenium polypyridyl complexes as apoptosis inducers in cancer cells, the cellular translocation and the important role of ROS-mediated signaling.Dalton Trans. 2014 Dec 7;43(45):17017-28. doi: 10.1039/c4dt01392a. Dalton Trans. 2014. PMID: 25087850
-
Copper(II) complexes containing hydrazone and bipyridine/phenanthroline ligands for anticancer application against breast cancer cells.J Inorg Biochem. 2025 Jan;262:112759. doi: 10.1016/j.jinorgbio.2024.112759. Epub 2024 Oct 12. J Inorg Biochem. 2025. PMID: 39426333
-
Synthesis, structure and anti-cancer activity of osmium complexes bearing π-bound arene substituents and phosphane Co-Ligands: A review.Eur J Med Chem. 2020 Sep 1;201:112483. doi: 10.1016/j.ejmech.2020.112483. Epub 2020 May 28. Eur J Med Chem. 2020. PMID: 32592914 Review.
-
Future potential of osmium complexes as anticancer drug candidates, photosensitizers and organelle-targeted probes.Dalton Trans. 2018 Oct 30;47(42):14841-14854. doi: 10.1039/c8dt03432j. Dalton Trans. 2018. PMID: 30325378 Review.
Cited by
-
High-Spin Iron(VI), Low-Spin Ruthenium(VI), and Magnetically Bistable Osmium(VI) in Molecular Group 8 Nitrido Trifluorides NMF3.Chemistry. 2021 Aug 11;27(45):11693-11700. doi: 10.1002/chem.202101404. Epub 2021 Jun 26. Chemistry. 2021. PMID: 34043842 Free PMC article.
-
Exploring the In Vivo and In Vitro Anticancer Activity of Rhenium Isonitrile Complexes.Inorg Chem. 2020 Jul 20;59(14):10285-10303. doi: 10.1021/acs.inorgchem.0c01442. Epub 2020 Jul 7. Inorg Chem. 2020. PMID: 32633531 Free PMC article.
-
Third row transition metals for the treatment of cancer.Philos Trans A Math Phys Eng Sci. 2015 Mar 13;373(2037):20140185. doi: 10.1098/rsta.2014.0185. Philos Trans A Math Phys Eng Sci. 2015. PMID: 25666060 Free PMC article. Review.
-
Enzymatic Assemblies Disrupt the Membrane and Target Endoplasmic Reticulum for Selective Cancer Cell Death.J Am Chem Soc. 2018 Aug 1;140(30):9566-9573. doi: 10.1021/jacs.8b04641. Epub 2018 Jul 24. J Am Chem Soc. 2018. PMID: 29995402 Free PMC article.
-
Synthesis and Assessment of Antibacterial Activities of Ruthenium(III) Mixed Ligand Complexes Containing 1,10-Phenanthroline and Guanide.Bioinorg Chem Appl. 2016;2016:3607924. doi: 10.1155/2016/3607924. Epub 2016 Oct 19. Bioinorg Chem Appl. 2016. PMID: 27833473 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

