Mechanistic basis for high stereoselectivity and broad substrate scope in the (salen)Co(III)-catalyzed hydrolytic kinetic resolution
- PMID: 24041239
- PMCID: PMC3875305
- DOI: 10.1021/ja408027p
Mechanistic basis for high stereoselectivity and broad substrate scope in the (salen)Co(III)-catalyzed hydrolytic kinetic resolution
Abstract
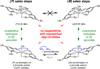
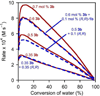
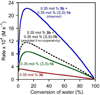
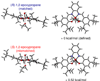
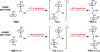
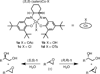
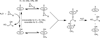
In the (salen)Co(III)-catalyzed hydrolytic kinetic resolution (HKR) of terminal epoxides, the rate- and stereoselectivity-determining epoxide ring-opening step occurs by a cooperative bimetallic mechanism with one Co(III) complex acting as a Lewis acid and another serving to deliver the hydroxide nucleophile. In this paper, we analyze the basis for the extraordinarily high stereoselectivity and broad substrate scope observed in the HKR. We demonstrate that the stereochemistry of each of the two (salen)Co(III) complexes in the rate-determining transition structure is important for productive catalysis: a measurable rate of hydrolysis occurs only if the absolute stereochemistry of each of these (salen)Co(III) complexes is the same. Experimental and computational studies provide strong evidence that stereochemical communication in the HKR is mediated by the stepped conformation of the salen ligand, and not the shape of the chiral diamine backbone of the ligand. A detailed computational analysis reveals that the epoxide binds the Lewis acidic Co(III) complex in a well-defined geometry imposed by stereoelectronic rather than steric effects. This insight serves as the basis of a complete stereochemical and transition structure model that sheds light on the reasons for the broad substrate generality of the HKR.
Figures




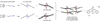


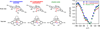


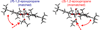


References
-
- Tokunaga M, Larrow JF, Kakiuchi F, Jacobsen EN. Science. 1997;277:936–938. - PubMed
- Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN. J. Am. Chem. Soc. 2002;124:1307–1315. - PubMed
- Stevenson CP, Nielsen LPC, Jacobsen EN, McKinley JD, White TD, Couturier MA, Ragan J. Org. Synth. 2006;83:162–169.
-
-
For reviews of applications of the HKR reaction in industrial and natural products synthesis, see: Larrow JF, Hemberger KE, Jasmin S, Kabir H, Morel P. Tetrahedron: Asymmetry. 2003;14:3589–3592. Schneider C. Synthesis. 2006:3919–3944. Kumar P, Naidu V, Gupta P. Tetrahedron. 2007;63:2745–2785. Furukawa Y, Suzuki T, Mikami M, Kitaori K, Yoshimoto H. J. Synth. Org. Chem. Japan. 2007;65:308–319. Kumar P, Gupta P. Synlett. 2009:1367–1382. Pellissier H. Adv. Synth. Catal. 2011;353:1613–1666.
-
-
-
Throughout this paper, we will refer to the property of the HKR catalysts to differentiate between substrate enantiomers as “stereoselectivity”. Stereoselectivity in any kinetic resolution is most unambiguously defined by the relative rate of reaction with the two enantiomers of the substrate (krel). We choose this term instead of “enantioselectivity”, which is applied more commonly to describe chiral catalysts, but generally refers to the enantiomer ratio (e.r.) or enantiomeric excess (e.e.) obtained from an achiral or rapidly-racemizing substrate.
-
-
-
(salen)Cr(III): Hansen KB, Leighton JL, Jacobsen EN. J. Am. Chem. Soc. 1996;118:10924–10925.
-
-
- Jacobsen EN. Acc. Chem. Res. 2000;33:421–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

