Cost-effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 infection in the United States
- PMID: 24041347
- PMCID: PMC3820000
- DOI: 10.1016/j.jval.2013.07.006
Cost-effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 infection in the United States
Abstract
Objectives: The phase 3 trial, Serine Protease Inhibitor Boceprevir and PegIntron/Rebetol-2 (RESPOND-2), demonstrated that the addition of boceprevir (BOC) to peginterferon-ribavirin (PR) resulted in significantly higher rates of sustained virologic response (SVR) in previously treated patients with chronic hepatitis C virus (HCV) genotype-1 infection as compared with PR alone. We evaluated the cost-effectiveness of treatment with BOC in previously treated patients with chronic hepatitis C in the United States using treatment-related data from RESPOND-2 and PROVIDE studies.
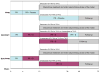
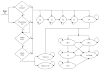
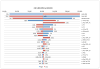
Methods: We developed a Markov cohort model to project the burden of HCV disease, lifetime costs, and quality-adjusted life-years associated with PR and two BOC-based therapies-response-guided therapy (BOC/RGT) and fixed-duration therapy for 48 weeks (BOC/PR48). We estimated treatment-related inputs (efficacy, adverse events, and discontinuations) from clinical trials and obtained disease progression rates, costs, and quality-of-life data from published studies. We estimated the incremental cost-effectiveness ratio (ICER) for BOC-based regimens as studied in RESPOND-2, as well as by patient's prior response to treatment and the IL-28B genotype.
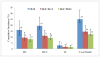
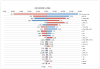
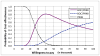
Results: BOC-based regimens were projected to reduce the lifetime incidence of liver-related complications by 43% to 53% in comparison with treatment with PR. The ICER of BOC/RGT in comparison with that of PR was $30,200, and the ICER of BOC/PR48 in comparison with that of BOC/RGT was $91,500. At a willingness-to-pay threshold of $50,000, the probabilities of BOC/RGT and BOC/PR48 being the preferred option were 0.74 and 0.25, respectively.
Conclusions: In patients previously treated for chronic HCV genotype-1 infection, BOC was projected to increase quality-adjusted life-years and reduce the lifetime incidence of liver complications. In addition, BOC-based therapies were projected to be cost-effective in comparison with PR alone at commonly used willingness-to-pay thresholds.
Keywords: Markov model; hepatitis C; protease inhibitor.
Copyright © 2013, International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Published by Elsevier Inc.
Conflict of interest statement
All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and report the following. Dr. Chhatwal is a former employee of Merck Sharp &Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ and has received consulting fees. Drs. Ferrante, El Khoury, Burroughs and Elbasha are current employees of Merck and hold stock and/or stock options. Dr. Brass is a former employee of Merck and holds stock and/or stock options. Dr. Bacon has received consultancy fees from Gilead, Three Rivers Pharmaceuticals, Valeant, Vertex, and Human Genome Sciences; has grants/grants pending from Roche, Gilead, Bristol Myers Squibb, Three Rivers Pharmaceuticals, Valeant, Vertex, Human Genome Sciences, Wyeth, and Romark Laboratories; payment for lectures including service on speakers bureaus for Three Rivers Pharmaceuticals, Gilead, and Merck; and served on Data and Safety Monitoring Boards for Novartis, ISIS, Vertex and Gilead. Dr Esteban is a member of the speaker’s bureau or is an advisor of Merck, Gilead, Novartis, Bristol-Myers Squibb and GlaxoSmithKline.
Figures
References
-
- Hepatitis C fact sheet. Geneva: World Health Organization; [Accessed December 8, 2012]. ( http://www.who.int/mediacentre/factsheets/fs164/en.)
-
- Ly KN, Xing J, Klevens RM, et al. The Increasing Burden of Mortality From Viral Hepatitis in the United States Between 1999 and 2007. Ann Intern Med. 2012;156:271–8. - PubMed
-
- Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007;42:513–21. - PubMed
-
- Camm C, Cabibbo G, Bronte F, et al. Retreatment with pegylated interferon plus ribavirin of chronic hepatitis C non-responders to interferon plus ribavirin: a meta-analysis. J Hepatol. 2009;51:675–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

