Variation in detection of ductal carcinoma in situ during screening mammography: a survey within the International Cancer Screening Network
- PMID: 24041876
- PMCID: PMC3874251
- DOI: 10.1016/j.ejca.2013.08.013
Variation in detection of ductal carcinoma in situ during screening mammography: a survey within the International Cancer Screening Network
Abstract
Background: There is concern about detection of ductal carcinoma in situ (DCIS) in screening mammography. DCIS accounts for a substantial proportion of screen-detected lesions but its effect on breast cancer mortality is debated. The International Cancer Screening Network conducted a comparative analysis to determine variation in DCIS detection.
Patients and methods: Data were collected during 2004-2008 on number of screening examinations, detected breast cancers, DCIS cases and Globocan 2008 breast cancer incidence rates derived from national or regional cancer registers. We calculated screen-detection rates for breast cancers and DCIS.
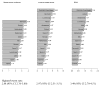
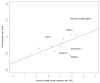
Results: Data were obtained from 15 screening settings in 12 countries; 7,176,050 screening examinations; 29,605 breast cancers and 5324 DCIS cases. The ratio between highest and lowest breast cancer incidence was 2.88 (95% confidence interval (CI) 2.76-3.00); 2.97 (95% CI 2.51-3.51) for detection of breast cancer; and 3.49 (95% CI 2.70-4.51) for detection of DCIS.
Conclusions: Considerable international variation was found in DCIS detection. This variation could not be fully explained by variation in incidence nor in breast cancer detection rates. It suggests the potential for wide discrepancies in management of DCIS resulting in overtreatment of indolent DCIS or undertreatment of potentially curable disease. Comprehensive cancer registration is needed to monitor DCIS detection. Efforts to understand discrepancies and standardise management may improve care.
Keywords: Breast cancer; Cancer registration; Ductal carcinoma in situ (DCIS); Screening mammography.
Copyright © 2013 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
No authors have declared a conflict of interest.
Figures
References
-
- Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–8. - PubMed
-
- [Accessed 11 July 2011]; http://www.breastscreening.cancer.gov/data/benchmarks/screening/2009/tab....
-
- Levi F, Te VC, Randimbison L, La Vecchia C. Trends of in situ carcinoma of the breast in Vaud, Switzerland. Eur J Cancer. 1997;33:903–6. - PubMed
-
- Barchielli A, Federico M, De Lisi V, Bucchi L, Ferretti S, Paci E, Ponti A, Buiatti E for the SCREENING working group. In situ breast cancer incidence trend and organised screening programmes in Italy. Eur J Cancer. 2005;41:1045–50. - PubMed
-
- Sørum R, Hofvind S, Skaane P, Haldorsen T. Trends in incidence of ductal carcinoma in situ: the effect of a population-based screening programme. Breast. 2010;19:499–505. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 CA063740/CA/NCI NIH HHS/United States
- U01CA69976/CA/NCI NIH HHS/United States
- U01 CA070040/CA/NCI NIH HHS/United States
- U01CA70013/CA/NCI NIH HHS/United States
- U01 CA086082/CA/NCI NIH HHS/United States
- U01CA63736/CA/NCI NIH HHS/United States
- U01CA86082/CA/NCI NIH HHS/United States
- Z99 CA999999/ImNIH/Intramural NIH HHS/United States
- U01CA70040/CA/NCI NIH HHS/United States
- U01CA63740/CA/NCI NIH HHS/United States
- U01 CA063731/CA/NCI NIH HHS/United States
- U01 CA086076/CA/NCI NIH HHS/United States
- U01 CA069976/CA/NCI NIH HHS/United States
- U01CA86076/CA/NCI NIH HHS/United States
- U01 CA063736/CA/NCI NIH HHS/United States
- U01 CA070013/CA/NCI NIH HHS/United States
- U01CA63731/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical