Protective humoral immunity elicited by a needle-free malaria vaccine comprised of a chimeric Plasmodium falciparum circumsporozoite protein and a Toll-like receptor 5 agonist, flagellin
- PMID: 24042110
- PMCID: PMC3837993
- DOI: 10.1128/IAI.00263-13
Protective humoral immunity elicited by a needle-free malaria vaccine comprised of a chimeric Plasmodium falciparum circumsporozoite protein and a Toll-like receptor 5 agonist, flagellin
Abstract
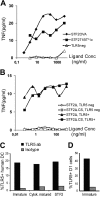
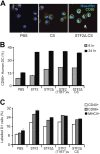
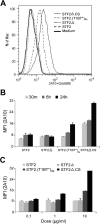
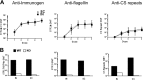
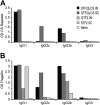
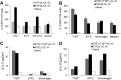
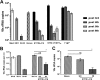
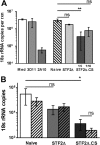
Immunization with Plasmodium sporozoites can elicit high levels of sterile immunity, and neutralizing antibodies from protected hosts are known to target the repeat region of the circumsporozoite (CS) protein on the parasite surface. CS-based subunit vaccines have been hampered by suboptimal immunogenicity and the requirement for strong adjuvants to elicit effective humoral immunity. Pathogen-associated molecular patterns (PAMPs) that signal through Toll-like receptors (TLRs) can function as potent adjuvants for innate and adaptive immunity. We examined the immunogenicity of recombinant proteins containing a TLR5 agonist, flagellin, and either full-length or selected epitopes of the Plasmodium falciparum CS protein. Mice immunized with either of the flagellin-modified CS constructs, administered intranasally (i.n.) or subcutaneously (s.c.), developed similar levels of malaria-specific IgG1 antibody and interleukin-5 (IL-5)-producing T cells. Importantly, immunization via the i.n. but not the s.c. route elicited sporozoite neutralizing antibodies capable of inhibiting >90% of sporozoite invasion in vitro and in vivo, as measured using a transgenic rodent parasite expressing P. falciparum CS repeats. These findings demonstrate that functional sporozoite neutralizing antibody can be elicited by i.n. immunization with a flagellin-modified P. falciparum CS protein and raise the potential of a scalable, safe, needle-free vaccine for the 40% of the world's population at risk of malaria.
Figures

Similar articles
-
Enhanced immunogenicity of Plasmodium falciparum peptide vaccines using a topical adjuvant containing a potent synthetic Toll-like receptor 7 agonist, imiquimod.Infect Immun. 2009 Feb;77(2):739-48. doi: 10.1128/IAI.00974-08. Epub 2008 Dec 1. Infect Immun. 2009. PMID: 19047411 Free PMC article.
-
Skin scarification with Plasmodium falciparum peptide vaccine using synthetic TLR agonists as adjuvants elicits malaria sporozoite neutralizing immunity.Sci Rep. 2016 Sep 14;6:32575. doi: 10.1038/srep32575. Sci Rep. 2016. PMID: 27624667 Free PMC article.
-
A linear peptide containing minimal T- and B-cell epitopes of Plasmodium falciparum circumsporozoite protein elicits protection against transgenic sporozoite challenge.Infect Immun. 2006 Dec;74(12):6929-39. doi: 10.1128/IAI.01151-06. Epub 2006 Oct 9. Infect Immun. 2006. PMID: 17030584 Free PMC article.
-
How to induce protective humoral immunity against Plasmodium falciparum circumsporozoite protein.J Exp Med. 2022 Feb 7;219(2):e20201313. doi: 10.1084/jem.20201313. Epub 2022 Jan 10. J Exp Med. 2022. PMID: 35006242 Free PMC article. Review.
-
T cell responses to repeat and non-repeat regions of the circumsporozoite protein detected in volunteers immunized with Plasmodium falciparum sporozoites.Mem Inst Oswaldo Cruz. 1992;87 Suppl 3:223-7. doi: 10.1590/s0074-02761992000700037. Mem Inst Oswaldo Cruz. 1992. PMID: 1364202 Review.
Cited by
-
Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands.Vaccines (Basel). 2014 Apr 25;2(2):323-53. doi: 10.3390/vaccines2020323. Vaccines (Basel). 2014. PMID: 26344622 Free PMC article. Review.
-
Computational Development of Transmission-Blocking Vaccine Candidates Based on Fused Antigens of Pre- and Post-fertilization Gametocytes Against Plasmodium falciparum.Bioinform Biol Insights. 2025 Mar 3;19:11779322241306215. doi: 10.1177/11779322241306215. eCollection 2025. Bioinform Biol Insights. 2025. PMID: 40034580 Free PMC article.
-
A porcine reproductive and respiratory syndrome virus (PRRSV) vaccine candidate based on the fusion protein of PRRSV glycoprotein 5 and the Toll-like Receptor-5 agonist Salmonella Typhimurium FljB.BMC Vet Res. 2015 May 23;11:121. doi: 10.1186/s12917-015-0439-0. BMC Vet Res. 2015. PMID: 26001608 Free PMC article.
-
DNA Vaccine-Encoded Flagellin Can Be Used as an Adjuvant Scaffold to Augment HIV-1 gp41 Membrane Proximal External Region Immunogenicity.Viruses. 2018 Feb 27;10(3):100. doi: 10.3390/v10030100. Viruses. 2018. PMID: 29495537 Free PMC article.
-
An unstable Th epitope of P. falciparum fosters central memory T cells and anti-CS antibody responses.PLoS One. 2014 Jul 1;9(7):e100639. doi: 10.1371/journal.pone.0100639. eCollection 2014. PLoS One. 2014. PMID: 24983460 Free PMC article.
References
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216:160–162 - PubMed
-
- Clyde DF. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 24:397–401 - PubMed
-
- Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207:71–73 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

