Osteocytes: master orchestrators of bone
- PMID: 24042263
- PMCID: PMC3947191
- DOI: 10.1007/s00223-013-9790-y
Osteocytes: master orchestrators of bone
Abstract
Osteocytes comprise the overwhelming majority of cells in bone and are its only true "permanent" resident cell population. In recent years, conceptual and technological advances on many fronts have helped to clarify the role osteocytes play in skeletal metabolism and the mechanisms they use to perform them. The osteocyte is now recognized as a major orchestrator of skeletal activity, capable of sensing and integrating mechanical and chemical signals from their environment to regulate both bone formation and resorption. Recent studies have established that the mechanisms osteocytes use to sense stimuli and regulate effector cells (e.g., osteoblasts and osteoclasts) are directly coupled to the environment they inhabit-entombed within the mineralized matrix of bone and connected to each other in multicellular networks. Communication within these networks is both direct (via cell-cell contacts at gap junctions) and indirect (via paracrine signaling by secreted signals). Moreover, the movement of paracrine signals is dependent on the movement of both solutes and fluid through the space immediately surrounding the osteocytes (i.e., the lacunar-canalicular system). Finally, recent studies have also shown that the regulatory capabilities of osteocytes extend beyond bone to include a role in the endocrine control of systemic phosphate metabolism. This review will discuss how a highly productive combination of experimental and theoretical approaches has managed to unearth these unique features of osteocytes and bring to light novel insights into the regulatory mechanisms operating in bone.
Conflict of interest statement
The authors have stated that they have no conflict of interest.
Figures
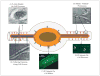
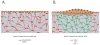

Comment in
-
Authors response to letter from Plotkin and Bellido.Calcif Tissue Int. 2014 Oct;95(4):384. doi: 10.1007/s00223-014-9906-z. Epub 2014 Aug 10. Calcif Tissue Int. 2014. PMID: 25108470 Free PMC article. No abstract available.
-
Comment on Osteocytes: masters orchestrators of bone.Calcif Tissue Int. 2014 Oct;95(4):382-3. doi: 10.1007/s00223-014-9905-0. Epub 2014 Aug 13. Calcif Tissue Int. 2014. PMID: 25116738 No abstract available.
References
-
- Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:176–190. - PubMed
-
- Palumbo C. A three-dimensional ultrastructural study of osteoid-osteocytes in the tibia of chick embryos. Cell and tissue research. 1986;246:125–131. - PubMed
-
- Palumbo C, Palazzini S, Zaffe D, Marotti G. Osteocyte differentiation in the tibia of newborn rabbit: an ultrastructural study of the formation of cytoplasmic processes. Acta anatomica. 1990;137:350–358. - PubMed
-
- Doty SB, Morey-Holton ER, Durnova GN, Kaplansky AS. Cosmos 1887: morphology, histochemistry, and vasculature of the growing rat tibia. FASEB J. 1990;4:16–23. - PubMed
-
- Dallas SL, Veno PA. Live imaging of bone cell and organ cultures. Methods Mol Biol. 2012;816:425–457. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

