K(ATP) channels and islet hormone secretion: new insights and controversies
- PMID: 24042324
- PMCID: PMC5890885
- DOI: 10.1038/nrendo.2013.166
K(ATP) channels and islet hormone secretion: new insights and controversies
Abstract
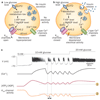
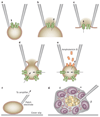
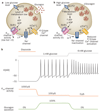
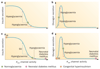
ATP-sensitive potassium channels (K(ATP) channels) link cell metabolism to electrical activity by controlling the cell membrane potential. They participate in many physiological processes but have a particularly important role in systemic glucose homeostasis by regulating hormone secretion from pancreatic islet cells. Glucose-induced closure of K(ATP) channels is crucial for insulin secretion. Emerging data suggest that K(ATP) channels also play a key part in glucagon secretion, although precisely how they do so remains controversial. This Review highlights the role of K(ATP) channels in insulin and glucagon secretion. We discuss how K(ATP) channels might contribute not only to the initiation of insulin release but also to the graded stimulation of insulin secretion that occurs with increasing glucose concentrations. The various hypotheses concerning the role of K(ATP) channels in glucagon release are also reviewed. Furthermore, we illustrate how mutations in K(ATP) channel genes can cause hyposecretion or hypersecretion of insulin, as in neonatal diabetes mellitus and congenital hyperinsulinism, and how defective metabolic regulation of the channel may underlie the hypoinsulinaemia and the hyperglucagonaemia that characterize type 2 diabetes mellitus. Finally, we outline how sulphonylureas, which inhibit K(ATP) channels, stimulate insulin secretion in patients with neonatal diabetes mellitus or type 2 diabetes mellitus, and suggest their potential use to target the glucagon secretory defects found in diabetes mellitus.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. - PubMed
-
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. - PubMed
-
- Zhang Q, et al. R-type Ca2+-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nat Cell Biol. 2007;9:453–460. - PubMed
-
- Gromada J, et al. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes. 2004;53(Suppl. 3):S181–S189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

