Oxetane synthesis through the Paternò-Büchi reaction
- PMID: 24043139
- PMCID: PMC6269742
- DOI: 10.3390/molecules180911384
Oxetane synthesis through the Paternò-Büchi reaction
Abstract
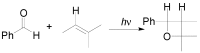
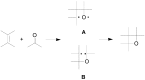
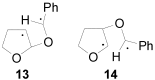
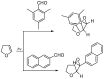
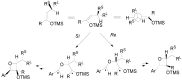
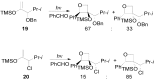
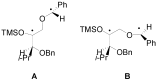
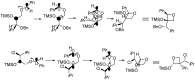
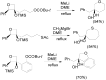
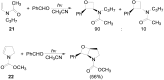
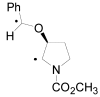
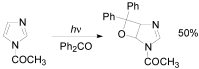
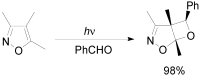
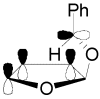
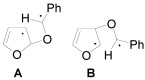
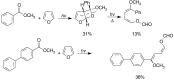
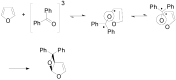
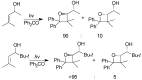
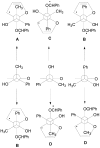
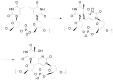
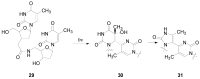
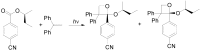
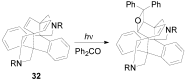
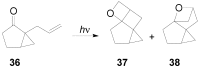
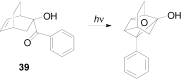
The Paternò-Büchi reaction is a photochemical reaction between a carbonyl compound and an alkene to give the corresponding oxetane. In this review the mechanism of the reaction is discussed. On this basis the described use in the reaction with electron rich alkenes (enolethers, enol esters, enol silyl ethers, enanines, heterocyclic compounds has been reported. The stereochemical behavior of the reaction is particularly stressed. We pointed out the reported applications of this reaction to the synthesis of naturally occuring compounds.
Figures

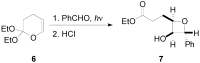

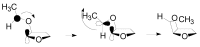
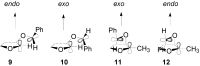
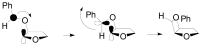

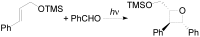
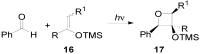
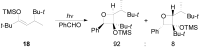
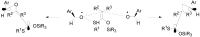

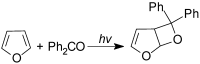
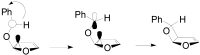

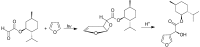
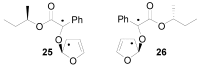
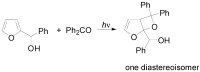
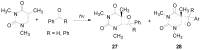
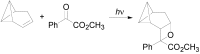
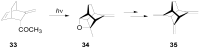
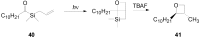
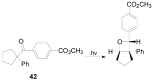
Similar articles
-
The Paternò-Büchi reaction - a comprehensive review.Photochem Photobiol Sci. 2019 Oct 9;18(10):2297-2362. doi: 10.1039/c9pp00148d. Photochem Photobiol Sci. 2019. PMID: 31273370
-
Visible-Light-Driven [2 + 2] Photocycloadditions between Benzophenone and C═C Bonds in Unsaturated Lipids.J Am Chem Soc. 2020 Feb 19;142(7):3499-3505. doi: 10.1021/jacs.9b12120. Epub 2020 Feb 11. J Am Chem Soc. 2020. PMID: 31994883
-
Visible-Light-Enabled Paternò-Büchi Reaction via Triplet Energy Transfer for the Synthesis of Oxetanes.Org Lett. 2020 Aug 21;22(16):6516-6519. doi: 10.1021/acs.orglett.0c02316. Epub 2020 Aug 11. Org Lett. 2020. PMID: 32806149
-
Synthesis of Azulene Derivatives from 2H-Cyclohepta[b]furan-2-ones as Starting Materials: Their Reactivity and Properties.Int J Mol Sci. 2021 Oct 1;22(19):10686. doi: 10.3390/ijms221910686. Int J Mol Sci. 2021. PMID: 34639027 Free PMC article. Review.
-
Synthesis of azetidines by aza Paternò-Büchi reactions.Chem Sci. 2020 Jun 12;11(29):7553-7561. doi: 10.1039/d0sc01017k. eCollection 2020 Aug 7. Chem Sci. 2020. PMID: 32832061 Free PMC article. Review.
Cited by
-
Strain Release Chemistry of Photogenerated Small-Ring Intermediates.Chemistry. 2021 Mar 8;27(14):4500-4516. doi: 10.1002/chem.202004178. Epub 2021 Jan 15. Chemistry. 2021. PMID: 33080091 Free PMC article. Review.
-
Oxetane-, Azetidine-, and Bicyclopentane-Bearing N-Heterocycles from Ynones: Scaffold Diversification via Ruthenium-Catalyzed Oxidative Alkynylation.Org Lett. 2023 Aug 11;25(31):5907-5910. doi: 10.1021/acs.orglett.3c02213. Epub 2023 Aug 1. Org Lett. 2023. PMID: 37527501 Free PMC article.
-
Chiral α-Stereogenic Oxetanols and Azetidinols via Alcohol-Mediated Reductive Coupling of Allylic Acetates: Enantiotopic π-Facial Selection in Symmetric Ketone Addition.ACS Catal. 2022 May 20;12(10):6172-6179. doi: 10.1021/acscatal.2c01647. Epub 2022 May 9. ACS Catal. 2022. PMID: 37063244 Free PMC article.
-
Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis.Chem Rev. 2016 Sep 14;116(17):9683-747. doi: 10.1021/acs.chemrev.5b00760. Epub 2016 Apr 27. Chem Rev. 2016. PMID: 27120289 Free PMC article. Review.
-
Light-Triggered Click Chemistry.Chem Rev. 2021 Jun 23;121(12):6991-7031. doi: 10.1021/acs.chemrev.0c00799. Epub 2020 Oct 26. Chem Rev. 2021. PMID: 33104332 Free PMC article. Review.
References
-
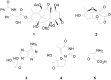
- Huang J., Yokoyama R., Yang C., Fukuyama Y. Merrilactone A, a novel neurotrophic sesquiterpene dilactone from Illicium merrillianum. Tetrahedron Lett. 2000;41:6111–6114. doi: 10.1016/S0040-4039(00)01023-6. - DOI
-
- Wang Y., Fleet G.W.J., Storer R., Myers P.L., Wallis C.J., Doherty O., Watkin D.J., Vogt K., Witty D.R., Wilson F.X., Peach J.M. Synthesis of the potent antiviral oxetane nucleoside epinoroxetanocin from D-lyxonolactone. Tetrahedron Asymmetry. 1990;1:527–530. doi: 10.1016/S0957-4166(00)80541-8. - DOI
-
- Yang C.O., Kurz W., Eugui E.M., Mc Roberts M.J., Verheyden J.P.H., Kurz L.J., Walker K.A.M. 4′-Substituted nucleosides as inhibitors of HIV: An unusual oxetane derivative. Tetrahedron Lett. 1992;33:41–44.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

