Caveolin-1 scaffolding domain promotes leukocyte adhesion by reduced basal endothelial nitric oxide-mediated ICAM-1 phosphorylation in rat mesenteric venules
- PMID: 24043249
- PMCID: PMC3840264
- DOI: 10.1152/ajpheart.00382.2013
Caveolin-1 scaffolding domain promotes leukocyte adhesion by reduced basal endothelial nitric oxide-mediated ICAM-1 phosphorylation in rat mesenteric venules
Abstract
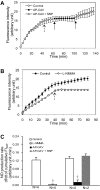
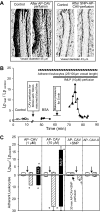
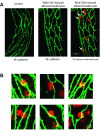
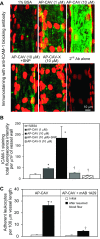
Exogenously applied caveolin-1 scaffolding domain (CAV) has been shown to inhibit inflammatory mediator-induced nitric oxide (NO) production and NO-mediated increases in microvessel permeability. However, the effect of CAV on endothelial basal NO that prevents leukocyte adhesion remains unknown. This study aims to investigate the roles of exogenously applied CAV in endothelial basal NO production, leukocyte adhesion, and adhesion-induced changes in microvessel permeability. Experiments were conducted in individually perfused rat mesenteric venules. Microvessel permeability was determined by measuring hydraulic conductivity (Lp). NO was quantified with fluorescence imaging in DAF-2-loaded vessels. Perfusing venules with CAV inhibited basal NO production without affecting basal Lp. Resuming blood flow in CAV-perfused vessels significantly increased leukocyte adhesion. The firmly adherent leukocytes altered neither basal Lp nor adherens junction integrity. Increases in Lp occurred only upon formyl-Met-Leu-Phe application that induces release of reactive oxygen species from the adherent leukocytes. The application of NO synthase inhibitor showed similar results to CAV, and NO donor abolished CAV-mediated leukocyte adhesion. Immunofluorescence staining showed increases in binding of ICAM-1 to an adhesion-blocking antibody concurrent with a Src-dependent ICAM-1 phosphorylation following CAV perfusion. Pre-perfusing vessels with anti-ICAM-1 blocking antibody or a Src kinase inhibitor attenuated CAV-induced leukocyte adhesion. These results indicate that the application of CAV, in addition to preventing excessive NO-mediated permeability increases, also causes reduction of basal NO and promotes ICAM-1-mediated leukocyte adhesion through Src activation-mediated ICAM-1 phosphorylation. CAV-induced leukocyte adhesion was uncoupled from leukocyte oxidative burst and microvessel barrier function, unless in the presence of a secondary stimulation.
Keywords: ICAM-1 phorsphorylation; caveolin-1; leukocyte adhesion; microvessel permeability; reactive oxygen species.
Figures



Similar articles
-
Endothelial [Ca2+]i and caveolin-1 antagonistically regulate eNOS activity and microvessel permeability in rat venules.Cardiovasc Res. 2010 Jul 15;87(2):340-7. doi: 10.1093/cvr/cvq006. Epub 2010 Jan 15. Cardiovasc Res. 2010. PMID: 20080986 Free PMC article.
-
Ischemia-reperfusion injury in rats affects hydraulic conductivity in two phases that are temporally and mechanistically separate.Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H2164-71. doi: 10.1152/ajpheart.00419.2008. Epub 2008 Sep 12. Am J Physiol Heart Circ Physiol. 2008. PMID: 18790838 Free PMC article.
-
H2O2-induced endothelial NO production contributes to vascular cell apoptosis and increased permeability in rat venules.Am J Physiol Heart Circ Physiol. 2013 Jan 1;304(1):H82-93. doi: 10.1152/ajpheart.00300.2012. Epub 2012 Oct 19. Am J Physiol Heart Circ Physiol. 2013. PMID: 23086988 Free PMC article.
-
Reactive species-induced microvascular dysfunction in ischemia/reperfusion.Free Radic Biol Med. 2019 May 1;135:182-197. doi: 10.1016/j.freeradbiomed.2019.02.031. Epub 2019 Mar 5. Free Radic Biol Med. 2019. PMID: 30849489 Free PMC article. Review.
-
Pharmacological targeting of ICAM-1 signaling in brain endothelial cells: potential for treating neuroinflammation.Cell Mol Neurobiol. 2005 Feb;25(1):153-70. doi: 10.1007/s10571-004-1380-0. Cell Mol Neurobiol. 2005. PMID: 15962512 Free PMC article. Review.
Cited by
-
Caveolin-1 mediates blood-brain barrier permeability, neuroinflammation, and cognitive impairment in SARS-CoV-2 infection.J Neuroimmunol. 2024 Mar 15;388:578309. doi: 10.1016/j.jneuroim.2024.578309. Epub 2024 Feb 4. J Neuroimmunol. 2024. PMID: 38335781 Free PMC article.
-
Reduction of Endothelial Nitric Oxide Increases the Adhesiveness of Constitutive Endothelial Membrane ICAM-1 through Src-Mediated Phosphorylation.Front Physiol. 2018 Jan 10;8:1124. doi: 10.3389/fphys.2017.01124. eCollection 2017. Front Physiol. 2018. PMID: 29367846 Free PMC article.
-
In vitro recapitulation of functional microvessels for the study of endothelial shear response, nitric oxide and [Ca2+]i.PLoS One. 2015 May 12;10(5):e0126797. doi: 10.1371/journal.pone.0126797. eCollection 2015. PLoS One. 2015. PMID: 25965067 Free PMC article.
-
Pulsatile stretch as a novel modulator of amyloid precursor protein processing and associated inflammatory markers in human cerebral endothelial cells.Sci Rep. 2018 Jan 26;8(1):1689. doi: 10.1038/s41598-018-20117-6. Sci Rep. 2018. PMID: 29374229 Free PMC article.
-
Caveolin-1 scaffolding domain peptides enhance anti-inflammatory effect of heme oxygenase-1 through interrupting its interact with caveolin-1.Oncotarget. 2017 Jun 20;8(25):40104-40114. doi: 10.18632/oncotarget.16676. Oncotarget. 2017. PMID: 28402952 Free PMC article.
References
-
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6: 1362–1367, 2000 - PubMed
-
- Cirino G, Fiorucci S, Sessa WC. Endothelial nitric oxide synthase: the Cinderella of inflammation? Trends Pharmacol Sci 24: 91–95, 2003 - PubMed
-
- Curry PE, Huxley VH, Sarelius IH. Techniques in microcirculation: measurement of permeability, pressure and flow. In: Cardiovascular Physiology. Techniques in the Life Sciences. New York: Elsevier, 1983, p. 1–34
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

