Removing sensory input disrupts spinal locomotor activity in the early postnatal period
- PMID: 24043359
- PMCID: PMC3898701
- DOI: 10.1007/s00359-013-0853-3
Removing sensory input disrupts spinal locomotor activity in the early postnatal period
Abstract
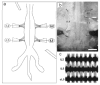
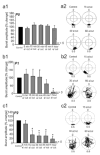
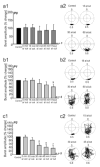
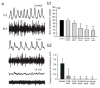
Motor patterns driving rhythmic movements of our lower limbs during walking are generated by groups of neurons within the spinal cord, called central pattern generators (CPGs). After suffering a spinal cord injury (SCI), many descending fibers from our brain are severed or become nonfunctional, leaving the spinal CPG network without its initiating drive. Recent studies have focused on the importance of maintaining sensory stimulation to the limbs of SCI patients as a way to initiate and control the CPG locomotor network. We began assessing the role of sensory feedback to the locomotor CPG network using a neonatal mouse spinal cord preparation where the hindlimbs are still attached. Removing sensory feedback coming from the hindlimbs by way of a lower lumbar transection or by ventral root denervation revealed a positive correlation in the ability of sensory input deprivation to disrupt ongoing locomotor activity on older versus younger animals. The differences in the motor responses as a function of age could be correlated with the loss of excitatory activity from sensory afferents. Continued studies on this field could eventually provide key information that translates into the design of novel therapeutic strategies to treat patients who have suffered a SCI.
Figures

Similar articles
-
Locomotor central pattern generator excitability states and serotonin sensitivity after spontaneous recovery from a neonatal lumbar spinal cord injury.Brain Res. 2019 Apr 1;1708:10-19. doi: 10.1016/j.brainres.2018.12.001. Epub 2018 Dec 4. Brain Res. 2019. PMID: 30521786
-
Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization.J Physiol. 2012 Oct 1;590(19):4735-59. doi: 10.1113/jphysiol.2012.240895. Epub 2012 Aug 6. J Physiol. 2012. PMID: 22869012 Free PMC article.
-
Rapid recovery and altered neurochemical dependence of locomotor central pattern generation following lumbar neonatal spinal cord injury.J Physiol. 2018 Jan 15;596(2):281-303. doi: 10.1113/JP274484. Epub 2017 Dec 3. J Physiol. 2018. PMID: 29086918 Free PMC article.
-
Probing the Human Spinal Locomotor Circuits by Phasic Step-Induced Feedback and by Tonic Electrical and Pharmacological Neuromodulation.Curr Pharm Des. 2017;23(12):1805-1820. doi: 10.2174/1381612822666161214144655. Curr Pharm Des. 2017. PMID: 27981912 Review.
-
Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord.Prog Neurobiol. 2003 Jul;70(4):347-61. doi: 10.1016/s0301-0082(03)00091-1. Prog Neurobiol. 2003. PMID: 12963092 Review.
Cited by
-
Genetically identified spinal interneurons integrating tactile afferents for motor control.J Neurophysiol. 2015 Dec;114(6):3050-63. doi: 10.1152/jn.00522.2015. Epub 2015 Oct 7. J Neurophysiol. 2015. PMID: 26445867 Free PMC article. Review.
-
Stepping responses to treadmill perturbations vary with severity of motor deficits in human SCI.J Neurophysiol. 2018 Aug 1;120(2):497-508. doi: 10.1152/jn.00486.2017. Epub 2018 Apr 18. J Neurophysiol. 2018. PMID: 29668389 Free PMC article.
References
-
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. - PubMed
-
- Barthe JY, Clarac F. Modulation of the spinal network for locomotion by substance P in the neonatal rat. Exp Brain Res. 1997;115:485–492. - PubMed
-
- Christenson J, Borman A, Lagerback PA, Grillner S. The dorsal cell, one class of primary sensory neurone in the lamprey spinal cord. I. Touch, pressure but no nociception-a physiological study. Brain Res. 1998;440:1–8. - PubMed
-
- Cragg JJ, Scott AL, Ramer MS. Depletion of spinal 5-HT accelerates mechanosensory recovery in the deafferented rat spinal cord. Exp Neurol. 2010;222:277–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

