Lysine metabolism in mammalian brain: an update on the importance of recent discoveries
- PMID: 24043460
- PMCID: PMC3838446
- DOI: 10.1007/s00726-013-1590-1
Lysine metabolism in mammalian brain: an update on the importance of recent discoveries
Abstract
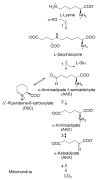
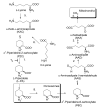
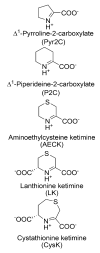
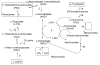
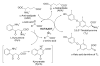
The lysine catabolism pathway differs in adult mammalian brain from that in extracerebral tissues. The saccharopine pathway is the predominant lysine degradative pathway in extracerebral tissues, whereas the pipecolate pathway predominates in adult brain. The two pathways converge at the level of ∆(1)-piperideine-6-carboxylate (P6C), which is in equilibrium with its open-chain aldehyde form, namely, α-aminoadipate δ-semialdehyde (AAS). A unique feature of the pipecolate pathway is the formation of the cyclic ketimine intermediate ∆(1)-piperideine-2-carboxylate (P2C) and its reduced metabolite L-pipecolate. A cerebral ketimine reductase (KR) has recently been identified that catalyzes the reduction of P2C to L-pipecolate. The discovery that this KR, which is capable of reducing not only P2C but also other cyclic imines, is identical to a previously well-described thyroid hormone-binding protein [μ-crystallin (CRYM)], may hold the key to understanding the biological relevance of the pipecolate pathway and its importance in the brain. The finding that the KR activity of CRYM is strongly inhibited by the thyroid hormone 3,5,3'-triiodothyronine (T3) has far-reaching biomedical and clinical implications. The inter-relationship between tryptophan and lysine catabolic pathways is discussed in the context of shared degradative enzymes and also potential regulation by thyroid hormones. This review traces the discoveries of enzymes involved in lysine metabolism in mammalian brain. However, there still remain unanswered questions as regards the importance of the pipecolate pathway in normal or diseased brain, including the nature of the first step in the pathway and the relationship of the pipecolate pathway to the tryptophan degradation pathway.
Figures
Similar articles
-
Insights into Enzyme Catalysis and Thyroid Hormone Regulation of Cerebral Ketimine Reductase/μ-Crystallin Under Physiological Conditions.Neurochem Res. 2015 Jun;40(6):1252-66. doi: 10.1007/s11064-015-1590-5. Epub 2015 May 1. Neurochem Res. 2015. PMID: 25931162
-
Reciprocal Control of Thyroid Binding and the Pipecolate Pathway in the Brain.Neurochem Res. 2017 Jan;42(1):217-243. doi: 10.1007/s11064-016-2015-9. Epub 2016 Aug 12. Neurochem Res. 2017. PMID: 27518089 Review.
-
Mouse lysine catabolism to aminoadipate occurs primarily through the saccharopine pathway; implications for pyridoxine dependent epilepsy (PDE).Biochim Biophys Acta Mol Basis Dis. 2017 Jan;1863(1):121-128. doi: 10.1016/j.bbadis.2016.09.006. Epub 2016 Sep 8. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27615426
-
Understanding cerebral L-lysine metabolism: the role of L-pipecolate metabolism in Gcdh-deficient mice as a model for glutaric aciduria type I.J Inherit Metab Dis. 2015 Mar;38(2):265-72. doi: 10.1007/s10545-014-9762-z. Epub 2014 Sep 12. J Inherit Metab Dis. 2015. PMID: 25214427
-
Lysine Catabolism Through the Saccharopine Pathway: Enzymes and Intermediates Involved in Plant Responses to Abiotic and Biotic Stress.Front Plant Sci. 2020 May 21;11:587. doi: 10.3389/fpls.2020.00587. eCollection 2020. Front Plant Sci. 2020. PMID: 32508857 Free PMC article. Review.
Cited by
-
Biochemical pathways supporting beta-lactam biosynthesis in the springtail Folsomia candida.Biol Open. 2016 Dec 15;5(12):1784-1789. doi: 10.1242/bio.019620. Biol Open. 2016. PMID: 27793835 Free PMC article.
-
Metabolic profile associated with distinct behavioral coping strategies of 129Sv and Bl6 mice in repeated motility test.Sci Rep. 2018 Feb 21;8(1):3405. doi: 10.1038/s41598-018-21752-9. Sci Rep. 2018. PMID: 29467440 Free PMC article.
-
Reshaping circadian metabolism in the suprachiasmatic nucleus and prefrontal cortex by nutritional challenge.Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29904-29913. doi: 10.1073/pnas.2016589117. Epub 2020 Nov 10. Proc Natl Acad Sci U S A. 2020. PMID: 33172990 Free PMC article.
-
Specialist and Generalist Fungal Parasites Induce Distinct Biochemical Changes in the Mandible Muscles of Their Host.Int J Mol Sci. 2019 Sep 17;20(18):4589. doi: 10.3390/ijms20184589. Int J Mol Sci. 2019. PMID: 31533250 Free PMC article.
-
Insights into Enzyme Catalysis and Thyroid Hormone Regulation of Cerebral Ketimine Reductase/μ-Crystallin Under Physiological Conditions.Neurochem Res. 2015 Jun;40(6):1252-66. doi: 10.1007/s11064-015-1590-5. Epub 2015 May 1. Neurochem Res. 2015. PMID: 25931162
References
-
- Akbarian S, Ruehl MG, Bliven E, Luiz LA, Peranelli AC, Baker SP, Roberts RC, Bunney WE, Jr, Conley RC, Jones EG, Tamminga CA, Guo Y. Chromatin alterations associated with down-regulated metabolic gene expression in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2005;62:829–840. - PubMed
-
- Aoki N, Ito K, Ito M. μ-Crystallin, thyroid hormone-binding protein, is expressed abundantly in the murine inner root sheath cells. J Invest Dermatol. 2000;115:402–405. - PubMed
-
- Arneson DW, Tipton RE, Ward JC. Hyperpipecolic acidemia. Occurrence in an infant with clinical findings of the cerebrohepatorenal (Zellweger) syndrome. Arch Neurol. 1982;39:713–716. - PubMed
-
- Aspen AJ, Meister A. The preparation and some properties of α-aminoadipic-δ-semialdehyde (Δ1-piperideine-6-carboxylic acid) Biochemistry. 1962a;1:600–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

