Prediction of survival by [18F]fluorodeoxyglucose positron emission tomography in patients with locally advanced non-small-cell lung cancer undergoing definitive chemoradiation therapy: results of the ACRIN 6668/RTOG 0235 trial
- PMID: 24043740
- PMCID: PMC3795891
- DOI: 10.1200/JCO.2012.47.5947
Prediction of survival by [18F]fluorodeoxyglucose positron emission tomography in patients with locally advanced non-small-cell lung cancer undergoing definitive chemoradiation therapy: results of the ACRIN 6668/RTOG 0235 trial
Abstract
Purpose: In this prospective National Cancer Institute-funded American College of Radiology Imaging Network/Radiation Therapy Oncology Group cooperative group trial, we hypothesized that standardized uptake value (SUV) on post-treatment [(18)F]fluorodeoxyglucose positron emission tomography (FDG-PET) correlates with survival in stage III non-small-cell lung cancer (NSCLC).
Patients and methods: Patients received conventional concurrent platinum-based chemoradiotherapy without surgery; postradiotherapy consolidation chemotherapy was allowed. Post-treatment FDG-PET was performed at approximately 14 weeks after radiotherapy. SUVs were analyzed both as peak SUV (SUVpeak) and maximum SUV (SUVmax; both institutional and central review readings), with institutional SUVpeak as the primary end point. Relationships between the continuous and categorical (cutoff) SUVs and survival were analyzed using Cox proportional hazards multivariate models.
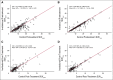
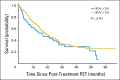
Results: Of 250 enrolled patients (226 were evaluable for pretreatment SUV), 173 patients were evaluable for post-treatment SUV analyses. The 2-year survival rate for the entire population was 42.5%. Pretreatment SUVpeak and SUVmax (mean, 10.3 and 13.1, respectively) were not associated with survival. Mean post-treatment SUVpeak and SUVmax were 3.2 and 4.0, respectively. Post-treatment SUVpeak was associated with survival in a continuous variable model (hazard ratio, 1.087; 95% CI, 1.014 to 1.166; P = .020). When analyzed as a prespecified binary value (≤ v > 3.5), there was no association with survival. However, in exploratory analyses, significant results for survival were found using an SUVpeak cutoff of 5.0 (P = .041) or 7.0 (P < .001). All results were similar when SUVmax was used in univariate and multivariate models in place of SUVpeak.
Conclusion: Higher post-treatment tumor SUV (SUVpeak or SUVmax) is associated with worse survival in stage III NSCLC, although a clear cutoff value for routine clinical use as a prognostic factor is uncertain at this time.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Prognostic impact of [18F]fluorodeoxyglucose positron emission tomography scanning in the era of molecular oncology.J Clin Oncol. 2014 May 20;32(15):1630. doi: 10.1200/JCO.2013.53.6300. Epub 2014 Apr 21. J Clin Oncol. 2014. PMID: 24752045 No abstract available.
-
Reply to J.-S. Ryu et al and A.T. Berman et al.J Clin Oncol. 2014 May 20;32(15):1632-3. doi: 10.1200/JCO.2013.54.6176. Epub 2014 Apr 21. J Clin Oncol. 2014. PMID: 24752050 No abstract available.
-
Predicting survival in non-small-cell lung cancer using positron emission tomography: several conclusions from multiple comparisons.J Clin Oncol. 2014 May 20;32(15):1631-2. doi: 10.1200/JCO.2013.54.3074. Epub 2014 Apr 21. J Clin Oncol. 2014. PMID: 24752052 No abstract available.
References
-
- Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. - PubMed
-
- Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. - PubMed
-
- Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28:3739–3745. - PubMed
-
- Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in unresectable non-small cell lung carcinoma. Lung Cancer. 1994;10(suppl 1):S239–S244. - PubMed
-
- Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–1292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

