Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas
- PMID: 24043741
- PMCID: PMC3867644
- DOI: 10.1200/JCO.2012.46.8793
Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas
Abstract
Purpose: Aurora A kinase (AAK) is overexpressed in aggressive lymphomas and can correlate with more histologically aggressive forms of disease. We therefore designed a phase II study of alisertib, a selective AAK inhibitor, in patients with relapsed and refractory aggressive non-Hodgkin lymphomas.
Patients and methods: Patients age ≥ 18 years were eligible if they had relapsed or refractory diffuse large B-cell lymphoma (DLBCL), mantle-cell lymphoma (MCL), transformed follicular lymphoma, Burkitt's lymphoma, or noncutaneous T-cell lymphoma. Alisertib was administered orally at 50 mg twice daily for 7 days in 21-day cycles.
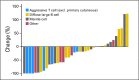
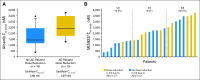
Results: We enrolled 48 patients. Histologies included DLBCL (n = 21), MCL (n = 13), peripheral T-cell lymphoma (n = 8), transformed follicular lymphoma (n = 5), and Burkitt's (n = 1). Most common grade 3 to 4 adverse events were neutropenia (63%), leukopenia (54%), anemia (35%), thrombocytopenia (33%), stomatitis (15%), febrile neutropenia (13%), and fatigue (6%). Four deaths during the study were attributed to progressive non-Hodgkin lymphoma (n = 2), treatment-related sepsis (n = 1), and unknown cause (n = 1). The overall response rate was 27%, including responses in three of 21 patients with DLBCL, three of 13 with MCL, one of one with Burkitt's lymphoma, two of five with transformed follicular lymphoma, and four of eight with noncutaneous T-cell lymphoma. The alisertib steady-state trough concentration (n = 25) revealed the expected pharmacokinetic variability, with a trend for higher incidence of adverse event-related dose reductions at higher trough concentrations. Analysis for AAK gene amplification and total AAK protein revealed no differences between histologies or correlation with clinical response.
Conclusion: The novel AAK inhibitor alisertib seems clinically active in both B- and T-cell aggressive lymphomas. On the basis of these results, confirmatory single-agent and combination studies have been initiated.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Aurora kinase inhibition as an anticancer strategy.J Clin Oncol. 2014 Jan 1;32(1):57-9. doi: 10.1200/JCO.2013.50.7988. Epub 2013 Sep 16. J Clin Oncol. 2014. PMID: 24043748 No abstract available.
References
-
- Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. - PubMed
-
- Ikezoe T, Takeuchi T, Yang J, et al. Analysis of aurora B kinase in non-Hodgkin lymphoma. Lab Invest. 2009;89:1364–1373. - PubMed
-
- Camacho E, Beà S, Salaverria I, et al. Analysis of aurora-A and hMPS1 mitotic kinases in mantle cell lymphoma. Int J Cancer. 2006;118:357–363. - PubMed
-
- Yakushijin Y, Hamada M, Yasukawa M. The expression of the aurora-A gene and its significance with tumorgenesis in non-Hodgkin's lymphoma. Leuk Lymphoma. 2004;45:1741–1746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

