Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study
- PMID: 24043745
- PMCID: PMC5950503
- DOI: 10.1200/JCO.2012.48.3552
Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study
Abstract
Purpose: The oral mammalian target of rapamycin inhibitor everolimus demonstrated promising efficacy in a phase II study of pretreated advanced gastric cancer. This international, double-blind, phase III study compared everolimus efficacy and safety with that of best supportive care (BSC) in previously treated advanced gastric cancer.
Patients and methods: Patients with advanced gastric cancer that progressed after one or two lines of systemic chemotherapy were randomly assigned to everolimus 10 mg/d (assignment schedule: 2:1) or matching placebo, both given with BSC. Randomization was stratified by previous chemotherapy lines (one v two) and region (Asia v rest of the world [ROW]). Treatment continued until disease progression or intolerable toxicity. Primary end point was overall survival (OS). Secondary end points included progression-free survival (PFS), overall response rate, and safety.
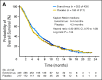
Results: Six hundred fifty-six patients (median age, 62.0 years; 73.6% male) were enrolled. Median OS was 5.4 months with everolimus and 4.3 months with placebo (hazard ratio, 0.90; 95% CI, 0.75 to 1.08; P = .124). Median PFS was 1.7 months and 1.4 months in the everolimus and placebo arms, respectively (hazard ratio, 0.66; 95% CI, 0.56 to 0.78). Common grade 3/4 adverse events included anemia, decreased appetite, and fatigue. The safety profile was similar in patients enrolled in Asia versus ROW.
Conclusion: Compared with BSC, everolimus did not significantly improve overall survival for advanced gastric cancer that progressed after one or two lines of previous systemic chemotherapy. The safety profile observed for everolimus was consistent with that observed for everolimus in other cancers.
Trial registration: ClinicalTrials.gov NCT00879333.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Jemal A Bray F Center MM, etal: Global cancer statistics CA Cancer J Clin 61:69–90,2011 - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer (including cancer in the proximal 5cm of the stomach), version 2.2012. http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
-
- Okines A Verheij M Allum W, etal: Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up Ann Oncol 21:v50–v54,2010suppl 5 - PubMed
-
- Sasako M Inoue M Lin JT, etal: Gastric Cancer Working Group report Jpn J Clin Oncol 40:i28–i37,2010suppl 1 - PubMed
-
- Howlander N Noone AM Krapcho M, etal: SEER Cancer Statistics Review, 1975-2008 http://seer.cancer.gov/csr/1975_2008
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

