Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time
- PMID: 24043751
- PMCID: PMC3795889
- DOI: 10.1200/JCO.2012.44.6716
Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time
Abstract
Purpose: Denosumab, an anti-RANK ligand monoclonal antibody, significantly increases bone metastasis-free survival (BMFS; hazard ratio [HR], 0.85; P = .028) and delays time to first bone metastasis in men with nonmetastatic castration-resistant prostate cancer (CRPC) and baseline prostate-specific antigen (PSA) ≥ 8.0 ng/mL and/or PSA doubling time (PSADT) ≤ 10.0 months. To identify men at greatest risk for bone metastasis or death, we evaluated relationships between PSA and PSADT with BMFS in the placebo group and the efficacy and safety of denosumab in men with PSADT ≤ 10, ≤ 6, and ≤ 4 months.
Patients and methods: A total of 1,432 men with nonmetastatic CRPC were randomly assigned 1:1 to monthly subcutaneous denosumab 120 mg or placebo. Enrollment began February 2006; primary analysis cutoff was July 2010, when approximately 660 men were anticipated to have developed bone metastases or died.
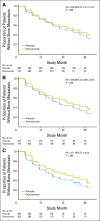
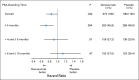
Results: In the placebo group, shorter BMFS was observed as PSADT decreased below 8 months. In analyses by shorter baseline PSADT, denosumab consistently increased BMFS by a median of 6.0, 7.2, and 7.5 months among men with PSADT ≤ 10 (HR, 0.84; P = .042), ≤ 6 (HR, 0.77; P = .006), and ≤ 4 months (HR, 0.71; P = .004), respectively. Denosumab also consistently increased time to bone metastasis by PSADT subset. No difference in survival was observed between treatment groups for the overall study population or PSADT subsets.
Conclusion: Patients with shorter PSADT are at greater risk for bone metastasis or death. Denosumab consistently improves BMFS in men with shorter PSADT and seems to have the greatest treatment effects in men at high risk for progression.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Re: Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time.J Urol. 2014 Aug;192(2):421-2. doi: 10.1016/j.juro.2014.05.077. Epub 2014 May 14. J Urol. 2014. PMID: 25034990 No abstract available.
References
-
- Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. - PubMed
-
- Nørgaard M, Jensen AØ, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–167. - PubMed
-
- Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: A population-based analysis of U.S. Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis. 2011;14:177–183. - PubMed
-
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

