In vivo adeno-associated viral vector-mediated genetic engineering of white and brown adipose tissue in adult mice
- PMID: 24043756
- PMCID: PMC3837045
- DOI: 10.2337/db13-0311
In vivo adeno-associated viral vector-mediated genetic engineering of white and brown adipose tissue in adult mice
Abstract
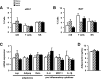
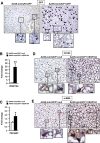
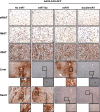
Adipose tissue is pivotal in the regulation of energy homeostasis through the balance of energy storage and expenditure and as an endocrine organ. An inadequate mass and/or alterations in the metabolic and endocrine functions of adipose tissue underlie the development of obesity, insulin resistance, and type 2 diabetes. To fully understand the metabolic and molecular mechanism(s) involved in adipose dysfunction, in vivo genetic modification of adipocytes holds great potential. Here, we demonstrate that adeno-associated viral (AAV) vectors, especially serotypes 8 and 9, mediated efficient transduction of white (WAT) and brown adipose tissue (BAT) in adult lean and obese diabetic mice. The use of short versions of the adipocyte protein 2 or uncoupling protein-1 promoters or micro-RNA target sequences enabled highly specific, long-term AAV-mediated transgene expression in white or brown adipocytes. As proof of concept, delivery of AAV vectors encoding for hexokinase or vascular endothelial growth factor to WAT or BAT resulted in increased glucose uptake or increased vessel density in targeted depots. This method of gene transfer also enabled the secretion of stable high levels of the alkaline phosphatase marker protein into the bloodstream by transduced WAT. Therefore, AAV-mediated genetic engineering of adipose tissue represents a useful tool for the study of adipose pathophysiology and, likely, for the future development of new therapeutic strategies for obesity and diabetes.
Figures
Similar articles
-
Overexpression of Adiponectin Receptor 1 Inhibits Brown and Beige Adipose Tissue Activity in Mice.Int J Mol Sci. 2021 Jan 18;22(2):906. doi: 10.3390/ijms22020906. Int J Mol Sci. 2021. PMID: 33477525 Free PMC article.
-
BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis.Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):E798-807. doi: 10.1073/pnas.1215236110. Epub 2013 Feb 6. Proc Natl Acad Sci U S A. 2013. PMID: 23388637 Free PMC article.
-
Adipocyte-specific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance.Mol Metab. 2016 Sep 28;5(12):1149-1161. doi: 10.1016/j.molmet.2016.09.009. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900258 Free PMC article.
-
CIDE Family-Mediated Unique Lipid Droplet Morphology in White Adipose Tissue and Brown Adipose Tissue Determines the Adipocyte Energy Metabolism.J Atheroscler Thromb. 2017 Oct 1;24(10):989-998. doi: 10.5551/jat.RV17011. Epub 2017 Sep 5. J Atheroscler Thromb. 2017. PMID: 28883211 Free PMC article. Review.
-
Mitochondria and endocrine function of adipose tissue.Best Pract Res Clin Endocrinol Metab. 2012 Dec;26(6):791-804. doi: 10.1016/j.beem.2012.06.002. Epub 2012 Jul 26. Best Pract Res Clin Endocrinol Metab. 2012. PMID: 23168280 Review.
Cited by
-
Cdo1 promotes PPARγ-mediated adipose tissue lipolysis in male mice.Nat Metab. 2022 Oct;4(10):1352-1368. doi: 10.1038/s42255-022-00644-3. Epub 2022 Oct 17. Nat Metab. 2022. PMID: 36253617
-
Specific and efficient gene knockout and overexpression in mouse interscapular brown adipocytes in vivo.STAR Protoc. 2022 Dec 16;3(4):101895. doi: 10.1016/j.xpro.2022.101895. Epub 2022 Dec 2. STAR Protoc. 2022. PMID: 36595932 Free PMC article.
-
Pancreatic transduction by helper-dependent adenoviral vectors via intraductal delivery.Hum Gene Ther. 2014 Sep;25(9):824-36. doi: 10.1089/hum.2013.182. Hum Gene Ther. 2014. PMID: 25046147 Free PMC article.
-
miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function.Mol Metab. 2016 Jun 15;5(8):615-625. doi: 10.1016/j.molmet.2016.06.005. eCollection 2016 Aug. Mol Metab. 2016. PMID: 27656399 Free PMC article.
-
Deubiquitinating enzyme USP2 regulates brown adipose tissue thermogenesis via controlling EBF2 stabilization.Mol Metab. 2025 Jun;96:102139. doi: 10.1016/j.molmet.2025.102139. Epub 2025 Apr 4. Mol Metab. 2025. PMID: 40189098 Free PMC article.
References
-
- Moller DE, Flier JS. Insulin resistance—mechanisms, syndromes, and implications. N Engl J Med 1991;325:938–948 - PubMed
-
- Spiegelman BM, Choy L, Hotamisligil GS, Graves RA, Tontonoz P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J Biol Chem 1993;268:6823–6826 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

