Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium
- PMID: 24043764
- PMCID: PMC3782042
- DOI: 10.1084/jem.20130903
Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium
Abstract
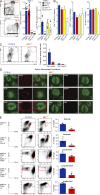
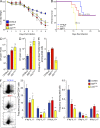
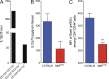
Dendritic cells (DCs), monocytes, and macrophages are closely related phagocytes that share many phenotypic features and, in some cases, a common developmental origin. Although the requirement for DCs in initiating adaptive immune responses is well appreciated, the role of monocytes and macrophages remains largely undefined, in part because of the lack of genetic tools enabling their specific depletion. Here, we describe a two-gene approach that requires overlapping expression of LysM and Csf1r to define and deplete monocytes and macrophages. The role of monocytes and macrophages in immunity to pathogens was tested by their selective depletion during infection with Citrobacter rodentium. Although neither cell type was required to initiate immunity, monocytes and macrophages contributed to the adaptive immune response by secreting IL-12, which induced Th1 polarization and IFN-γ secretion. Thus, whereas DCs are indispensable for priming naive CD4(+) T cells, monocytes and macrophages participate in intestinal immunity by producing mediators that direct T cell polarization.
Figures




Similar articles
-
IL-23-mediated mononuclear phagocyte crosstalk protects mice from Citrobacter rodentium-induced colon immunopathology.Nat Commun. 2015 Mar 12;6:6525. doi: 10.1038/ncomms7525. Nat Commun. 2015. PMID: 25761673 Free PMC article.
-
Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by beta-chemokines rather than IL-12.J Immunol. 2000 Oct 15;165(8):4388-96. doi: 10.4049/jimmunol.165.8.4388. J Immunol. 2000. PMID: 11035076
-
CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium.Mucosal Immunol. 2013 Jan;6(1):177-88. doi: 10.1038/mi.2012.61. Epub 2012 Aug 1. Mucosal Immunol. 2013. PMID: 22854708 Free PMC article.
-
Intestinal macrophages and dendritic cells: what's the difference?Trends Immunol. 2014 Jun;35(6):270-7. doi: 10.1016/j.it.2014.04.003. Epub 2014 Apr 30. Trends Immunol. 2014. PMID: 24794393 Review.
-
Blood monocytes: development, heterogeneity, and relationship with dendritic cells.Annu Rev Immunol. 2009;27:669-92. doi: 10.1146/annurev.immunol.021908.132557. Annu Rev Immunol. 2009. PMID: 19132917 Review.
Cited by
-
Ikaros Inhibits Group 3 Innate Lymphoid Cell Development and Function by Suppressing the Aryl Hydrocarbon Receptor Pathway.Immunity. 2016 Jul 19;45(1):185-97. doi: 10.1016/j.immuni.2016.06.027. Immunity. 2016. PMID: 27438771 Free PMC article.
-
Th17 Immunity in the Colon Is Controlled by Two Novel Subsets of Colon-Specific Mononuclear Phagocytes.Front Immunol. 2021 Apr 28;12:661290. doi: 10.3389/fimmu.2021.661290. eCollection 2021. Front Immunol. 2021. PMID: 33995384 Free PMC article.
-
Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice.JCI Insight. 2018 Sep 6;3(17):e120220. doi: 10.1172/jci.insight.120220. eCollection 2018 Sep 6. JCI Insight. 2018. PMID: 30185672 Free PMC article.
-
Tissue adaptation: Implications for gut immunity and tolerance.J Exp Med. 2017 May 1;214(5):1211-1226. doi: 10.1084/jem.20162014. Epub 2017 Apr 21. J Exp Med. 2017. PMID: 28432200 Free PMC article. Review.
-
Oral administration of taheebo (Tabebuia avellanedae Lorentz ex Griseb.) water extract prevents DSS-induced colitis in mice by up-regulating type II T helper immune responses.BMC Complement Altern Med. 2017 Sep 6;17(1):448. doi: 10.1186/s12906-017-1952-4. BMC Complement Altern Med. 2017. PMID: 28877696 Free PMC article.
References
-
- Athie-Morales V., Smits H.H., Cantrell D.A., Hilkens C.M. 2004. Sustained IL-12 signaling is required for Th1 development. J. Immunol. 172:61–69 - PubMed
-
- Auffray C., Fogg D.K., Narni-Mancinelli E., Senechal B., Trouillet C., Saederup N., Leemput J., Bigot K., Campisi L., Abitbol M., et al. 2009. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J. Exp. Med. 206:595–606 10.1084/jem.20081385 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

