Programmed cell death 1 inhibits inflammatory helper T-cell development through controlling the innate immune response
- PMID: 24043779
- PMCID: PMC3791721
- DOI: 10.1073/pnas.1315828110
Programmed cell death 1 inhibits inflammatory helper T-cell development through controlling the innate immune response
Abstract
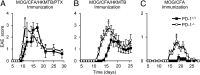
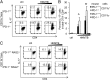
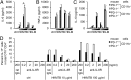
Programmed cell death 1 (PD-1) is an inhibitory coreceptor on immune cells and is essential for self-tolerance because mice genetically lacking PD-1 (PD-1(-/-)) develop spontaneous autoimmune diseases. PD-1(-/-) mice are also susceptible to severe experimental autoimmune encephalomyelitis (EAE), characterized by a massive production of effector/memory T cells against myelin autoantigen, the mechanism of which is not fully understood. We found that an increased primary response of PD-1(-/-) mice to heat-killed mycobacteria (HKMTB), an adjuvant for EAE, contributed to the enhanced production of T-helper 17 (Th17) cells. Splenocytes from HKMTB-immunized, lymphocyte-deficient PD-1(-/-) recombination activating gene (RAG)2(-/-) mice were found to drive antigen-specific Th17 cell differentiation more efficiently than splenocytes from HKMTB-immunized PD-1(+/+) RAG2(-/-) mice. This result suggested PD-1's involvement in the regulation of innate immune responses. Mice reconstituted with PD-1(-/-) RAG2(-/-) bone marrow and PD-1(+/+) CD4(+) T cells developed more severe EAE compared with the ones reconstituted with PD-1(+/+) RAG2(-/-) bone marrow and PD-1(+/+) CD4(+) T cells. We found that upon recognition of HKMTB, CD11b(+) macrophages from PD-1(-/-) mice produced very high levels of IL-6, which helped promote naive CD4(+) T-cell differentiation into IL-17-producing cells. We propose a model in which PD-1 negatively regulates antimycobacterial responses by suppressing innate immune cells, which in turn prevents autoreactive T-cell priming and differentiation to inflammatory effector T cells.
Keywords: autoimmunity; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: From discovery to clinical application. Int Immunol. 2007;19(7):813–824. - PubMed
-
- Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–322. - PubMed
-
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. - PubMed
-
- Wang J, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–452. - PubMed
-
- Prokunina L, et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32(4):666–669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

