ECHIDNA-mediated post-Golgi trafficking of auxin carriers for differential cell elongation
- PMID: 24043780
- PMCID: PMC3791722
- DOI: 10.1073/pnas.1309057110
ECHIDNA-mediated post-Golgi trafficking of auxin carriers for differential cell elongation
Abstract
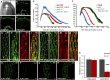
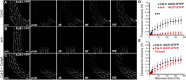
The plant hormone indole-acetic acid (auxin) is essential for many aspects of plant development. Auxin-mediated growth regulation typically involves the establishment of an auxin concentration gradient mediated by polarly localized auxin transporters. The localization of auxin carriers and their amount at the plasma membrane are controlled by membrane trafficking processes such as secretion, endocytosis, and recycling. In contrast to endocytosis or recycling, how the secretory pathway mediates the localization of auxin carriers is not well understood. In this study we have used the differential cell elongation process during apical hook development to elucidate the mechanisms underlying the post-Golgi trafficking of auxin carriers in Arabidopsis. We show that differential cell elongation during apical hook development is defective in Arabidopsis mutant echidna (ech). ECH protein is required for the trans-Golgi network (TGN)-mediated trafficking of the auxin influx carrier AUX1 to the plasma membrane. In contrast, ech mutation only marginally perturbs the trafficking of the highly related auxin influx carrier LIKE-AUX1-3 or the auxin efflux carrier PIN-FORMED-3, both also involved in hook development. Electron tomography reveals that the trafficking defects in ech mutant are associated with the perturbation of secretory vesicle genesis from the TGN. Our results identify differential mechanisms for the post-Golgi trafficking of de novo-synthesized auxin carriers to plasma membrane from the TGN and reveal how trafficking of auxin influx carriers mediates the control of differential cell elongation in apical hook development.
Keywords: IAA; morphogenesis; sorting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sabatini S, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99(5):463–472. - PubMed
-
- Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115(5):591–602. - PubMed
-
- Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426(6963):147–153. - PubMed
-
- Grieneisen V-A, Xu J, Marée A-F, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449(7165):1008–1013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

