Actin-related protein2/3 complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation
- PMID: 24043783
- PMCID: PMC3791730
- DOI: 10.1073/pnas.1308419110
Actin-related protein2/3 complex regulates tight junctions and terminal differentiation to promote epidermal barrier formation
Abstract
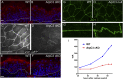
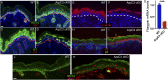
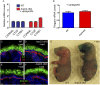
The epidermis provides an essential seal from the external environment and retains fluids within the body. To form an effective barrier, cells in the epidermis must form tight junctions and terminally differentiate into cornified envelopes. Here, we demonstrate that the branched actin nucleator, the actin-related protein (Arp)2/3 complex, is unexpectedly required for both these activities. Loss of the ArpC3 subunit of the Arp2/3 complex resulted in minimal changes in the morphogenesis and architecture of this stratified squamous epithelium, but resulted in profound defects in its physiology. Mutant embryos did not develop an effective barrier to the external environment and died within hours of birth. We discovered two underlying causes for these effects. First, ArpC3 was essential for robust assembly and function of tight junctions, specialized cell-cell adhesions that restrict water loss in the epidermis. Second, there were defects in differentiation of the epidermis and the production of cornified envelopes, structures essential for barrier activity. Underlying this defect, we found that YAP was inappropriately active not only in the ArpC3 mutant tissue, but also in cultured cells. Inhibition of YAP activity rescued the differentiation and barrier defects caused by loss of ArpC3. These results demonstrate previously unappreciated roles for the Arp2/3 complex and highlight the functions of branched actin networks in a complex tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol. 2013;14(1):7–12. - PubMed
-
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. - PubMed
-
- Robinson RC, et al. Crystal structure of Arp2/3 complex. Science. 2001;294(5547):1679–1684. - PubMed
-
- Volkmann N, et al. Structure of Arp2/3 complex in its activated state and in actin filament branch junctions. Science. 2001;293(5539):2456–2459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

