GPR171 is a hypothalamic G protein-coupled receptor for BigLEN, a neuropeptide involved in feeding
- PMID: 24043826
- PMCID: PMC3791715
- DOI: 10.1073/pnas.1312938110
GPR171 is a hypothalamic G protein-coupled receptor for BigLEN, a neuropeptide involved in feeding
Abstract
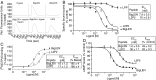
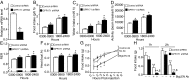
Multiple peptide systems, including neuropeptide Y, leptin, ghrelin, and others, are involved with the control of food intake and body weight. The peptide LENSSPQAPARRLLPP (BigLEN) has been proposed to act through an unknown receptor to regulate body weight. In the present study, we used a combination of ligand-binding and receptor-activity assays to characterize a Gαi/o protein-coupled receptor activated by BigLEN in the mouse hypothalamus and Neuro2A cells. We then selected orphan G protein-coupled receptors expressed in the hypothalamus and Neuro2A cells and tested each for activation by BigLEN. G protein-coupled receptor 171 (GPR171) is activated by BigLEN, but not by the C terminally truncated peptide LittleLEN. The four C-terminal amino acids of BigLEN are sufficient to bind and activate GPR171. Overexpression of GPR171 leads to an increase, and knockdown leads to a decrease, in binding and signaling by BigLEN and the C-terminal peptide. In the hypothalamus GPR171 expression complements the expression of BigLEN, and its level and activity are elevated in mice lacking BigLEN. In mice, shRNA-mediated knockdown of hypothalamic GPR171 leads to a decrease in BigLEN signaling and results in changes in food intake and metabolism. The combination of GPR171 shRNA together with neutralization of BigLEN peptide by antibody absorption nearly eliminates acute feeding in food-deprived mice. Taken together, these results demonstrate that GPR171 is the BigLEN receptor and that the BigLEN-GPR171 system plays an important role in regulating responses associated with feeding and metabolism in mice.
Keywords: NPY/AgRP; deorphanization; neuroendocrine peptide; orexigenic; proSAAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Identification of a small-molecule ligand that activates the neuropeptide receptor GPR171 and increases food intake.Sci Signal. 2016 May 31;9(430):ra55. doi: 10.1126/scisignal.aac8035. Sci Signal. 2016. PMID: 27245612 Free PMC article.
-
The BigLEN-GPR171 Peptide Receptor System Within the Basolateral Amygdala Regulates Anxiety-Like Behavior and Contextual Fear Conditioning.Neuropsychopharmacology. 2017 Dec;42(13):2527-2536. doi: 10.1038/npp.2017.79. Epub 2017 Apr 20. Neuropsychopharmacology. 2017. PMID: 28425495 Free PMC article.
-
Opioid-Induced Signaling and Antinociception Are Modulated by the Recently Deorphanized Receptor, GPR171.J Pharmacol Exp Ther. 2019 Oct;371(1):56-62. doi: 10.1124/jpet.119.259242. Epub 2019 Jul 15. J Pharmacol Exp Ther. 2019. PMID: 31308196 Free PMC article.
-
Orphan neuropeptides and receptors: Novel therapeutic targets.Pharmacol Ther. 2018 May;185:26-33. doi: 10.1016/j.pharmthera.2017.11.006. Epub 2017 Nov 22. Pharmacol Ther. 2018. PMID: 29174650 Free PMC article. Review.
-
Neuropeptide PEN and Its Receptor GPR83: Distribution, Signaling, and Regulation.ACS Chem Neurosci. 2019 Apr 17;10(4):1884-1891. doi: 10.1021/acschemneuro.8b00559. Epub 2019 Feb 21. ACS Chem Neurosci. 2019. PMID: 30726666 Free PMC article. Review.
Cited by
-
Identification of a small-molecule ligand that activates the neuropeptide receptor GPR171 and increases food intake.Sci Signal. 2016 May 31;9(430):ra55. doi: 10.1126/scisignal.aac8035. Sci Signal. 2016. PMID: 27245612 Free PMC article.
-
Evaluating functional ligand-GPCR interactions in cell-based assays.Methods Cell Biol. 2021;166:15-42. doi: 10.1016/bs.mcb.2021.06.006. Epub 2021 Jul 29. Methods Cell Biol. 2021. PMID: 34752330 Free PMC article.
-
Identification of prognostic biomarkers for breast cancer brain metastases based on the bioinformatics analysis.Biochem Biophys Rep. 2022 Jan 10;29:101203. doi: 10.1016/j.bbrep.2022.101203. eCollection 2022 Mar. Biochem Biophys Rep. 2022. PMID: 35059509 Free PMC article.
-
Carboxypeptidases in disease: insights from peptidomic studies.Proteomics Clin Appl. 2014 Jun;8(5-6):327-37. doi: 10.1002/prca.201300090. Epub 2014 Mar 24. Proteomics Clin Appl. 2014. PMID: 24470285 Free PMC article. Review.
-
The role of orphan G protein-coupled receptors in pain.Heliyon. 2024 Mar 26;10(7):e28818. doi: 10.1016/j.heliyon.2024.e28818. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38590871 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

