Direct interaction of eag domains and cyclic nucleotide-binding homology domains regulate deactivation gating in hERG channels
- PMID: 24043860
- PMCID: PMC3787778
- DOI: 10.1085/jgp.201310995
Direct interaction of eag domains and cyclic nucleotide-binding homology domains regulate deactivation gating in hERG channels
Abstract
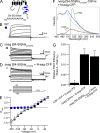
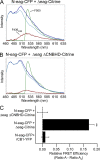
Human ether-á-go-go (eag)-related gene (hERG) potassium channels play a critical role in cardiac repolarization and are characterized by unusually slow closing (deactivation) kinetics. The N-terminal "eag" domain and a C-terminal C-linker/cyclic nucleotide-binding homology domain (CNBHD) are required for regulation of slow deactivation. The region between the S4 and S5 transmembrane domains (S4-S5 linker) is also implicated in this process, but the mechanism for regulation of slow deactivation is unclear. Here, using an eag domain-deleted channel (hERG Δeag) fused to Citrine fluorescent protein, we found that most channels bearing individual alanine mutations in the S4-S5 linker were directly regulated by recombinant eag domains fused to a cyan fluorescent protein (N-eag-CFP) and had robust Förster resonance energy transfer (FRET). Additionally, a channel bearing a group of eight alanine residues in the S4-S5 linker was not measurably regulated by N-eag-CFP domains, but robust FRET was measured. These findings demonstrate that the eag domain associated with all of the S4-S5 linker mutant channels. In contrast, channels that also lacked the CNBHD (hERG Δeag ΔCNBHD-Citrine) were not measurably regulated by N-eag-CFP nor was FRET detected, suggesting that the C-linker/CNBHD was required for eag domains to directly associate with the channel. In a FRET hybridization assay, N-eag-CFP had robust FRET with a C-linker/CNBHD-Citrine, suggesting a direct and specific interaction between the eag domain and the C-linker/CNBHD. Lastly, coexpression of a hERG subunit lacking the CNBHD and the distal C-terminal region (hERG ΔpCT-Citrine) with hERG Δeag-CFP subunits had FRET and partial restoration of slow deactivation. Collectively, these findings reveal that the C-linker/CNBHD, but not the S4-S5 linker, was necessary for the eag domain to associate with the channel, that the eag domain and the C-linker/CNBHD were sufficient for a direct interaction, and that an intersubunit interaction between the eag domain and the C-linker/CNBHD regulated slow deactivation in hERG channels at the plasma membrane.
Figures







Comment in
-
Domain-domain interactions in ion channels.J Gen Physiol. 2013 Oct;142(4):347-50. doi: 10.1085/jgp.201311090. Epub 2013 Sep 16. J Gen Physiol. 2013. PMID: 24043858 Free PMC article. No abstract available.
Similar articles
-
Two mutations at different positions in the CNBH domain of the hERG channel accelerate deactivation and impair the interaction with the EAG domain.J Physiol. 2018 Oct;596(19):4629-4650. doi: 10.1113/JP276208. Epub 2018 Sep 3. J Physiol. 2018. PMID: 30086184 Free PMC article.
-
Insight into the molecular interaction between the cyclic nucleotide-binding homology domain and the eag domain of the hERG channel.FEBS Lett. 2014 Aug 25;588(17):2782-8. doi: 10.1016/j.febslet.2014.05.056. Epub 2014 Jun 12. FEBS Lett. 2014. PMID: 24931372
-
The structural mechanism of KCNH-channel regulation by the eag domain.Nature. 2013 Sep 19;501(7467):444-8. doi: 10.1038/nature12487. Epub 2013 Aug 25. Nature. 2013. PMID: 23975098 Free PMC article.
-
HERG potassium channel regulation by the N-terminal eag domain.Cell Signal. 2012 Aug;24(8):1592-8. doi: 10.1016/j.cellsig.2012.04.004. Epub 2012 Apr 13. Cell Signal. 2012. PMID: 22522181 Free PMC article. Review.
-
Gating and regulation of KCNH (ERG, EAG, and ELK) channels by intracellular domains.Channels (Austin). 2020 Dec;14(1):294-309. doi: 10.1080/19336950.2020.1816107. Channels (Austin). 2020. PMID: 32924766 Free PMC article. Review.
Cited by
-
Revealing a hidden conducting state by manipulating the intracellular domains in KV10.1 exposes the coupling between two gating mechanisms.Elife. 2024 Sep 11;12:RP91420. doi: 10.7554/eLife.91420. Elife. 2024. PMID: 39259196 Free PMC article.
-
Hysteretic hERG channel gating current recorded at physiological temperature.Sci Rep. 2022 Apr 8;12(1):5950. doi: 10.1038/s41598-022-10003-7. Sci Rep. 2022. PMID: 35396394 Free PMC article.
-
LRMP inhibits cAMP potentiation of HCN4 channels by disrupting intramolecular signal transduction.bioRxiv [Preprint]. 2024 Jan 24:2023.08.29.555242. doi: 10.1101/2023.08.29.555242. bioRxiv. 2024. Update in: Elife. 2024 Apr 23;12:RP92411. doi: 10.7554/eLife.92411. PMID: 37693562 Free PMC article. Updated. Preprint.
-
Development of a High-Throughput Flow Cytometry Assay to Monitor Defective Trafficking and Rescue of Long QT2 Mutant hERG Channels.Front Physiol. 2018 Apr 19;9:397. doi: 10.3389/fphys.2018.00397. eCollection 2018. Front Physiol. 2018. PMID: 29725305 Free PMC article.
-
Schizophrenia-Associated hERG channel Kv11.1-3.1 Exhibits a Unique Trafficking Deficit that is Rescued Through Proteasome Inhibition for High Throughput Screening.Sci Rep. 2016 Feb 16;6:19976. doi: 10.1038/srep19976. Sci Rep. 2016. PMID: 26879421 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

