Cholera toxin directly enhances IL-17A production from human CD4+ T cells
- PMID: 24043897
- PMCID: PMC3825190
- DOI: 10.4049/jimmunol.1301079
Cholera toxin directly enhances IL-17A production from human CD4+ T cells
Abstract
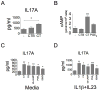
The significance of Th17 cells and IL-17A signaling in host defense and disease development has been demonstrated in various infection and autoimmune models. Additionally, the generation of Th17 cells is highly influenced by microbes. However, the specific bacterial components capable of shaping Th17 responses have not been well defined. The goals of this study were to understand how a bacterial toxin, cholera toxin (CT), modulates Th17-dominated response in isolated human CD4(+) T cells, and what are the mechanisms associated with this modulation. CD4(+) cells isolated from human peripheral blood were treated with CT. The levels of cytokine production and specific Th cell responses were determined by ELISA, Luminex assay, and flow cytometry. Along with the decreased production of other proinflammatory cytokines (IFN-γ, TNF-α, and IL-2), we found that CT could directly enhance the IL-17A production through a cAMP-dependent pathway. This enhancement is specific for IL-17A but not for IL-17F, IL-22, and CCL20. Interestingly, CT could increase IL-17A production only from Th17-committed cells, such as CCR6(+)CD4(+) T cells and in vitro-differentiated Th17 cells. Furthermore, we also demonstrated that this direct effect occurs at a transcriptional level because CT stimulates the reporter activity in Jurkat and primary CD4(+) T cells transfected with the IL-17A promoter-reporter construct. This study shows that CT has the capacity to directly shape Th17 responses in the absence of APCs. Our findings highlight the potentials of bacterial toxins in the regulation of human Th17 responses.
Conflict of interest statement
Figures






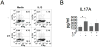
References
-
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature immunology. 2007;8:950–957. - PubMed
-
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nature reviews Immunology. 2008;8:337–348. - PubMed
-
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. The Journal of experimental medicine. 2007;204:2803–2812. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

