Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis
- PMID: 24045232
- PMCID: PMC3801438
- DOI: 10.1038/emboj.2013.204
Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis
Abstract
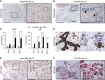
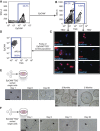
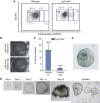
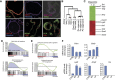
Lgr5 marks adult stem cells in multiple adult organs and is a receptor for the Wnt-agonistic R-spondins (RSPOs). Intestinal, stomach and liver Lgr5(+) stem cells grow in 3D cultures to form ever-expanding organoids, which resemble the tissues of origin. Wnt signalling is inactive and Lgr5 is not expressed under physiological conditions in the adult pancreas. However, we now report that the Wnt pathway is robustly activated upon injury by partial duct ligation (PDL), concomitant with the appearance of Lgr5 expression in regenerating pancreatic ducts. In vitro, duct fragments from mouse pancreas initiate Lgr5 expression in RSPO1-based cultures, and develop into budding cyst-like structures (organoids) that expand five-fold weekly for >40 weeks. Single isolated duct cells can also be cultured into pancreatic organoids, containing Lgr5 stem/progenitor cells that can be clonally expanded. Clonal pancreas organoids can be induced to differentiate into duct as well as endocrine cells upon transplantation, thus proving their bi-potentiality.
Conflict of interest statement
MH, TS and HC are inventors on a patent application related to this work.
Figures



References
-
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36 - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 - PubMed
-
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R (2001) Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 128: 5109–5117 - PubMed
-
- Blaydon DC, Ishii Y, O'Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu-Havu VK, Tidman N, Moss C, Watson R, de Berker D, Wajid M, Christiano AM, Kelsell DP (2006) The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet 38: 1245–1247 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

