Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: involvement of the VEGF-MMP pathway
- PMID: 24045404
- PMCID: PMC3887344
- DOI: 10.1038/jcbfm.2013.163
Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: involvement of the VEGF-MMP pathway
Abstract
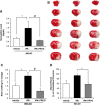
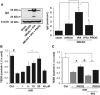
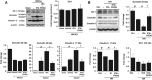
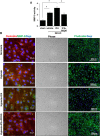
Tissue plasminogen activator (tPA) is the only FDA-approved treatment for acute stroke, but its use remains limited. Progesterone (PROG) has shown neuroprotection in ischemia, but before clinical testing, we must determine how it affects hemorrhagic transformation in tPA-treated ischemic rats. Male Sprague-Dawley rats underwent middle cerebral artery occlusion with reperfusion at 4.5 hours and tPA treatment at 4.5 hours, or PROG treatment intraperitoneally at 2 hours followed by subcutaneous injection at 6 hours post occlusion. Rats were killed at 24 hours and brains evaluated for cerebral hemorrhage, swelling, blood-brain barrier (BBB) permeability, and levels of matrix metalloproteinase-9 (MMP-9), vascular endothelial growth factor level (VEGF), and tight junction (TJ) proteins. We also evaluated PROG's efficacy in preventing tPA-induced impairment of transendothelial electrical resistance (TEER) and TJ proteins under hypoxia/reoxygenation in the endothelial cells. Delayed tPA treatment induced significant hemorrhagic conversion and brain swelling. Treatment with PROG plus tPA ameliorated hemorrhage, hemispheric swelling, BBB permeability, MMP-9 induction, and VEGF levels compared with controls. Progesterone treatment significantly prevented tPA-induced decrease in TEER and expression of occludin and claudin-5, and attenuated VEGF levels in culture media subjected to hypoxia. The study concluded that PROG may extend the time window for tPA administration in ischemic stroke and reduce hemorrhagic conversion.
Figures

References
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. - PubMed
-
- Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat Rev Neurol. 2011;7:400–409. - PubMed
-
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. - PubMed
-
- Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci. 2007;25:151–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

