Non-Darwinian dynamics in therapy-induced cancer drug resistance
- PMID: 24045430
- PMCID: PMC4657953
- DOI: 10.1038/ncomms3467
Non-Darwinian dynamics in therapy-induced cancer drug resistance
Abstract
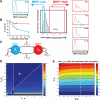
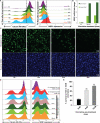
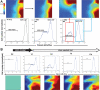
The development of drug resistance, the prime cause of failure in cancer therapy, is commonly explained by the selection of resistant mutant cancer cells. However, dynamic non-genetic heterogeneity of clonal cell populations continuously produces metastable phenotypic variants (persisters), some of which represent stem-like states that confer resistance. Even without genetic mutations, Darwinian selection can expand these resistant variants, which would explain the invariably rapid emergence of stem-like resistant cells. Here, by using quantitative measurements and modelling, we show that appearance of multidrug resistance in HL60 leukemic cells following treatment with vincristine is not explained by Darwinian selection but by Lamarckian induction. Single-cell longitudinal monitoring confirms the induction of multidrug resistance in individual cells. Associated transcriptome changes indicate a lasting stress response consistent with a drug-induced switch between high-dimensional cancer attractors. Resistance induction correlates with Wnt pathway upregulation and is suppressed by β-catenin knockdown, revealing a new opportunity for early therapeutic intervention against the development of drug resistance.
Figures
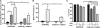
References
-
- Chabner BA, Roberts TGJ. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5:65–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

