A peptide probe for targeted brown adipose tissue imaging
- PMID: 24045463
- PMCID: PMC3806199
- DOI: 10.1038/ncomms3472
A peptide probe for targeted brown adipose tissue imaging
Abstract
The presence of brown adipose tissue responsible for thermogenic energy dissipation has been revealed in adult humans and has high clinical importance. Owing to limitations of current methods for brown adipose tissue detection, analysing the abundance and localization of brown adipose tissue in the body has remained challenging. Here we screen a combinatorial peptide library in mice and characterize a peptide (with the sequence CPATAERPC) that selectively binds to the vascular endothelium of brown adipose tissue, but not of intraperitoneal white adipose tissue. We show that in addition to brown adipose tissue, this peptide probe also recognizes the vasculature of brown adipose tissue-like depots of subcutaneous white adipose tissue. Our results indicate that the CPATAERPC peptide localizes to brown adipose tissue even in the absence of sympathetic nervous system stimulation. Finally, we demonstrate that this probe can be used to identify brown adipose tissue depots in mice by whole-body near-infrared fluorescence imaging.
Figures
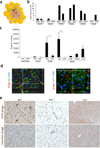

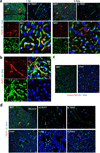
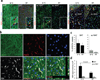

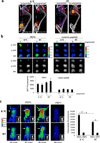
References
-
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. - PubMed
-
- Enerback S. The origins of brown adipose tissue. N. Engl. J. Med. 2009;360:2021–2023. - PubMed
-
- Tam CS, Lecoultre V, Ravussin E. Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation. 2012;125:2782–2791. - PubMed
-
- Paidisetty S, Blodgett TM. Brown fat: atypical locations and appearances encountered in PET/CT. Am. J. Roentgenol. 2009;193:359–366. - PubMed
-
- Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

