Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury
- PMID: 24045574
- PMCID: PMC3945733
- DOI: 10.1038/mi.2013.63
Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury
Abstract
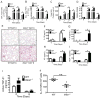
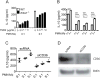
Mononuclear phagocyte recognition of apoptotic cells triggering suppressive cytokine signaling is a key event in inflammation resolution from injury. Mice deficient in thrombospondin (TSP)-1 (thbs1⁻/⁻), an extracellular matrix glycoprotein that bridges cell-cell interactions, are prone to lipopolysaccharide-induced lung injury and show defective macrophage interleukin (IL)-10 production during the resolution phase of inflammation. Reconstitution of IL-10 rescues thbs1⁻/⁻ mice from persistent neutrophilic lung inflammation and injury and thbs1⁻/⁻ alveolar macrophages show defective IL-10 production following intratracheal instillation of apoptotic neutrophils despite intact efferocytosis. Following co-culture with apoptotic neutrophils, thbs1⁻/⁻ macrophages show a selective defect in IL-10 production, whereas prostaglandin E2 and transforming growth factor beta 1 responses remain intact. Full macrophage IL-10 responses require the engagement of TSP-1 structural repeat 2 domain and the macrophage scavenger receptor CD36 LIMP-II Emp sequence homology (CLESH) domain in vitro. Although TSP-1 is not essential for macrophage engulfment of apoptotic neutrophils in vivo, TSP-1 aids in the curtailment of inflammatory responses during the resolution phase of injury in the lungs by providing a means by which apoptotic cells are recognized and trigger optimal IL-10 production by macrophages.
Conflict of interest statement
Figures



References
-
- Fowler AA, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98:593–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

