Mean overall survival gain with aflibercept plus FOLFIRI vs placebo plus FOLFIRI in patients with previously treated metastatic colorectal cancer
- PMID: 24045663
- PMCID: PMC3790175
- DOI: 10.1038/bjc.2013.523
Mean overall survival gain with aflibercept plus FOLFIRI vs placebo plus FOLFIRI in patients with previously treated metastatic colorectal cancer
Abstract
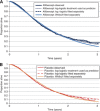
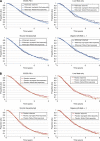
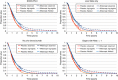
Background: Mean survival in cancer trials can be estimated with statistical techniques to extrapolate study survival curves. This methodology was applied to data from the VELOUR trial, where use of the novel biologic aflibercept (ziv-aflibercept in the United States) in combination with fluorouracil+leucovorin+irinotecan (FOLFIRI), had significantly increased median overall survival (OS) by 1.44 months, vs placebo plus FOLFIRI in patients with metastatic colorectal cancer (mCRC) resistant to, or that had progressed following, an oxaliplatin-containing regimen.
Methods: Parametric survival analyses were used to identify distributions with the best fit to the empirical VELOUR data. Mean OS for the two treatment groups (and pre-defined subgroups) was calculated from the fitted curves over a 15-year survival period.
Results: Overall, the log-logistic distribution was the best-fitting for both treatment arms and, with it, the estimated difference in mean OS over 15 years between aflibercept+FOLFIRI and placebo+FOLFIRI was 4.7 months. In addition, the survival advantage with aflibercept was at least 3 months for the ITT population, whichever distribution was used to extrapolate survival.
Conclusion: Extrapolation of survival curves suggests the mean OS difference for aflibercept in the VELOUR trial is at least 3 months in the ITT population and selected subgroups.
Figures

References
-
- Adam R, Wicherts DA, de Haas RJ, Ciacio O, Levi F, Paule B, Ducreux M, Azoulay D, Bismuth H, Castaing D. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure. J Clin Oncol. 2009;27 (11:1829–1835. - PubMed
-
- Boag J.1948The presentation and analysis of the result of radiotherapy Br J Radiol 21(244189passim. - PubMed
-
- Boag J. Maximum likelihood estimates of the proportion of patients cured by cancer therapy. J Roy Stat Soc Ser B (Methodological) 1949;11:15–53.
-
- Chapman JA, Lickley HL, Trudeau ME, Hanna WM, Kahn HJ, Murray D, Sawka CA, Mobbs BG, McCready DR, Pritchard KI. Ascertaining prognosis for breast cancer in node-negative patients with innovative survival analysis. Breast J. 2006;12 (1:37–47. - PubMed
-
- Christopherson R, James KE, Tableman M, Marshall P, Johnson FE. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107 (1:325–332. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

