Randomised phase II study of S-1/cisplatin plus TSU-68 vs S-1/cisplatin in patients with advanced gastric cancer
- PMID: 24045669
- PMCID: PMC3798959
- DOI: 10.1038/bjc.2013.555
Randomised phase II study of S-1/cisplatin plus TSU-68 vs S-1/cisplatin in patients with advanced gastric cancer
Erratum in
- Br J Cancer. 2014 Dec 9;111(12):2382
Abstract
Background: This study aimed to determine whether combination S-1 plus cisplatin (CDDP) therapy, the most widely used therapy for Japanese patients with advanced gastric cancer, and the novel oral antiangiogenic agent TSU-68 could contribute to gastric cancer treatment.
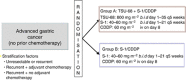
Methods: Ninety-three patients with chemotherapy-naïve unresectable or recurrent advanced gastric cancers were randomised into two groups: TSU-68 plus S-1/CDDP (group A) and S-1/CDDP (group B) groups. Both patient groups received identical S-1 and CDDP dosages. TSU-68 was orally administered for 35 consecutive days. Group B patients received S-1 orally twice daily for three consecutive weeks, followed by intravenous CDDP on day 8. The primary endpoint was progression-free survival (PFS).
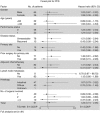
Results: Median PFS periods were 208 and 213 days in groups A and B, respectively (P=0.427). Median survival periods for groups A and B were 497.0 and 463.5 days, respectively (P=0.219). No statistically significant differences were noted for PFS, survival or the adverse event (AE) incidence rate. All AEs were expected according to previous reports for TSU-68, TS-1, and CDDP.
Conclusion: Combination therapy involving TSU-68, S-1, and CDDP was safe and well tolerated in patients with chemotherapy-naïve unresectable or recurrent advanced gastric cancers. However, factors related to therapeutic efficacy should be investigated further.
Figures


References
-
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. - PubMed
-
- Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu J, Ohtsu A, Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–1069. - PubMed
-
- Fuchs CS, Tomasek J, Cho JY, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Tabernero J, Zalcberg JR, Chau I, Koshiji M, Hsu Y, Schwartz JD, Ajani JA.2013REGARD: A phase III, randomized, double-blinded trial of ramucirumab and best supportive care (BSC) versus placebo and BSC in the treatment of metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma following disease progression on first-line platinum- and/or fluoropyrimidine-containing combination therapy J Clin Oncol 31(4_supplLBA5.
-
- Inaba F, Kanai F, Aramaki T, Yamamoto T, Tanaka T, Yamakado K, Kaneko S, Kudo M, Imanaka K, Kora S, Nishida N, Kawai N, Seki H, Matsui O, Arioka H, Arai Y. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolization. Eur J Cancer. 2013;49:2832–2840. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

