Efficacy of yoga for vasomotor symptoms: a randomized controlled trial
- PMID: 24045673
- PMCID: PMC3871975
- DOI: 10.1097/GME.0b013e31829e4baa
Efficacy of yoga for vasomotor symptoms: a randomized controlled trial
Abstract
Objective: This study aims to determine the efficacy of yoga in alleviating vasomotor symptoms (VMS) frequency and bother.
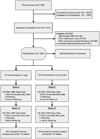
Methods: This study was a three-by-two factorial, randomized controlled trial. Eligible women were randomized to yoga (n = 107), exercise (n = 106), or usual activity (n = 142), and were simultaneously randomized to a double-blind comparison of ω-3 fatty acid (n = 177) or placebo (n = 178) capsules. Yoga intervention consisted of 12 weekly 90-minute yoga classes with daily home practice. Primary outcomes were VMS frequency and bother assessed by daily diaries at baseline, 6 weeks, and 12 weeks. Secondary outcomes included insomnia symptoms (Insomnia Severity Index) at baseline and 12 weeks.
Results: Among 249 randomized women, 237 (95%) completed 12-week assessments. The mean baseline VMS frequency was 7.4 per day (95% CI, 6.6 to 8.1) in the yoga group and 8.0 per day (95% CI, 7.3 to 8.7) in the usual activity group. Intent-to-treat analyses included all participants with response data (n = 237). There was no difference between intervention groups in the change in VMS frequency from baseline to 6 and 12 weeks (mean difference [yoga--usual activity] from baseline at 6 wk, -0.3 [95% CI, -1.1 to 0.5]; mean difference [yoga--usual activity] from baseline at 12 wk, -0.3 [95% CI, -1.2 to 0.6]; P = 0.119 across both time points). Results were similar for VMS bother. At week 12, yoga was associated with an improvement in insomnia symptoms (mean difference [yoga - usual activity] in the change in Insomnia Severity Index, 1.3 [95% CI, -2.5 to -0.1]; P = 0.007).
Conclusions: Among healthy women, 12 weeks of yoga class plus home practice, compared with usual activity, do not improve VMS frequency or bother but reduce insomnia symptoms.
Trial registration: ClinicalTrials.gov NCT01178892.
Conflict of interest statement
Dr. Newton received research support from Integrated Diagnostics Inc. Dr. Learman is a consultant on a Data Monitoring Committee for Ariosa Diagnostics. Dr. Freeman has received research support from Forest Laboratories, Inc., and Bionovo, Inc. Dr. Cohen has been a consultant for Noven pharmaceutical, and has received research support from: Astra-Zeneca Pharmaceuticals; Bristol-Myers Squibb; Cephalon, Inc.; GlaxoSmithKline; Ortho-McNeil Janssen; Pfizer Inc.; and Sunovion Pharmaceuticals, Inc. Dr. Joffe has received grant support from Cephalon/Teva, is on the advisory board for Noven, and has done consulting for Sunovion. Dr. Ensrud is a consultant on a Data Monitoring Committee for Merck, Sharp & Dohme. Dr. All other authors have no direct conflicts of interest or financial disclosures relevant to this manuscript.
Figures
Comment in
-
Menopause strategies: Finding Lasting Answers for Symptoms and Health: eliminating hot flashes--still not a slam dunk!Menopause. 2014 Apr;21(4):321-2. doi: 10.1097/GME.0000000000000217. Menopause. 2014. PMID: 24552975 No abstract available.
-
Three alternative ways to treat VMS failed.Climacteric. 2014 Aug;17(4):512-3. Climacteric. 2014. PMID: 25147889 No abstract available.
References
-
- Anderson GL, Limacher M, Assaf AR, et al. Effects of Conjugated Equine Estrogen in Postmenopausal Women with Hysterectomy: The Women's Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701–1712. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results from the Women's Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. - PubMed
-
- Carmody J, Crawford S, Churchill L. A Pilot Study of Mindfulness-Based Stress Reduction for Hot Flashes. Menopause. 2006;13:760–769. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U01AG032659/AG/NIA NIH HHS/United States
- U01 AG032682/AG/NIA NIH HHS/United States
- U01 AG032659/AG/NIA NIH HHS/United States
- U01AG032699/AG/NIA NIH HHS/United States
- R01 AG048209/AG/NIA NIH HHS/United States
- U01AG032700/AG/NIA NIH HHS/United States
- U01 AG032699/AG/NIA NIH HHS/United States
- U01AG032669/AG/NIA NIH HHS/United States
- U01 AG032656/AG/NIA NIH HHS/United States
- U01 AG032700/AG/NIA NIH HHS/United States
- U01AG032682/AG/NIA NIH HHS/United States
- UL1RR02571/RR/NCRR NIH HHS/United States
- U01 AG032669/AG/NIA NIH HHS/United States
- U01AG032656/AG/NIA NIH HHS/United States
- UL1 TR000371/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical