Dhrs3 protein attenuates retinoic acid signaling and is required for early embryonic patterning
- PMID: 24045938
- PMCID: PMC3814744
- DOI: 10.1074/jbc.M113.514984
Dhrs3 protein attenuates retinoic acid signaling and is required for early embryonic patterning
Abstract
All-trans-retinoic acid (atRA) is an important morphogen involved in many developmental processes, including neural differentiation, body axis formation, and organogenesis. During early embryonic development, atRA is synthesized from all-trans-retinal (atRAL) in an irreversible reaction mainly catalyzed by retinal dehydrogenase 2 (aldh1a2), whereas atRAL is converted from all-trans-retinol via reversible oxidation by retinol dehydrogenases, members of the short-chain dehydrogenase/reductase family. atRA is degraded by cytochrome P450, family 26 (cyp26). We have previously identified a short-chain dehydrogenase/reductase 3 (dhrs3), which showed differential expression patterns in Xenopus embryos. We show here that the expression of dhrs3 was induced by atRA treatment and overexpression of Xenopus nodal related 1 (xnr1) in animal cap assay. Overexpression of dhrs3 enhanced the phenotype of excessive cyp26a1. In embryos overexpressing aldh1a2 or retinol dehydrogenase 10 (rdh10) in the presence of their respective substrates, Dhrs3 counteracted the action of Aldh1a2 or Rdh10, indicating that retinoic acid signaling is attenuated. Knockdown of Dhrs3 by antisense morpholino oligonucleotides resulted in a phenotype of shortened anteroposterior axis, reduced head structure, and perturbed somitogenesis, which were also found in embryos treated with an excess of atRA. Examination of the expression of brachyury, not, goosecoid, and papc indicated that convergent extension movement was defective in Dhrs3 morphants. Taken together, these studies suggest that dhrs3 participates in atRA metabolism by reducing atRAL levels and is required for proper anteroposterior axis formation, neuroectoderm patterning, and somitogenesis.
Keywords: Development; Embryo; Gene Regulation; Retinal Metabolism; Retinoid; dhrs3, Retinoic Acid, Embryonic Patterning, Xenopus.
Figures
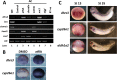




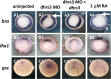
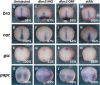

Similar articles
-
Retinol dehydrogenase 10 is a feedback regulator of retinoic acid signalling during axis formation and patterning of the central nervous system.Development. 2009 Feb;136(3):461-72. doi: 10.1242/dev.024901. Development. 2009. PMID: 19141675
-
The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development.FASEB J. 2013 Dec;27(12):4877-89. doi: 10.1096/fj.13-227967. Epub 2013 Sep 4. FASEB J. 2013. PMID: 24005908 Free PMC article.
-
Developmental expression of Xenopus short-chain dehydrogenase/reductase 3.Int J Dev Biol. 2010;54(8-9):1355-60. doi: 10.1387/ijdb.092984rk. Int J Dev Biol. 2010. PMID: 20563993 Free PMC article.
-
Involvement of alcohol dehydrogenase, short-chain dehydrogenase/reductase, aldehyde dehydrogenase, and cytochrome P450 in the control of retinoid signaling by activation of retinoic acid synthesis.Biochemistry. 1996 Sep 24;35(38):12221-7. doi: 10.1021/bi961176+. Biochemistry. 1996. PMID: 8823154 Review.
-
Enzymatic Metabolism of Vitamin A in Developing Vertebrate Embryos.Nutrients. 2016 Dec 15;8(12):812. doi: 10.3390/nu8120812. Nutrients. 2016. PMID: 27983671 Free PMC article. Review.
Cited by
-
Generation of Retinaldehyde for Retinoic Acid Biosynthesis.Biomolecules. 2019 Dec 18;10(1):5. doi: 10.3390/biom10010005. Biomolecules. 2019. PMID: 31861321 Free PMC article. Review.
-
Identification and characterization of short-chain dehydrogenase/reductase 3 (DHRS3) deficiency, a retinoic acid embryopathy of humans.Genet Med Open. 2025 Mar 29;3:103427. doi: 10.1016/j.gimo.2025.103427. eCollection 2025. Genet Med Open. 2025. PMID: 40519748 Free PMC article.
-
Mechanisms of Feedback Regulation of Vitamin A Metabolism.Nutrients. 2022 Mar 21;14(6):1312. doi: 10.3390/nu14061312. Nutrients. 2022. PMID: 35334970 Free PMC article. Review.
-
Retinoic Acid Organizes the Zebrafish Vagus Motor Topographic Map via Spatiotemporal Coordination of Hgf/Met Signaling.Dev Cell. 2020 May 4;53(3):344-357.e5. doi: 10.1016/j.devcel.2020.03.017. Epub 2020 Apr 16. Dev Cell. 2020. PMID: 32302545 Free PMC article.
-
New insights and changing paradigms in the regulation of vitamin A metabolism in development.Wiley Interdiscip Rev Dev Biol. 2017 May;6(3):10.1002/wdev.264. doi: 10.1002/wdev.264. Epub 2017 Feb 16. Wiley Interdiscip Rev Dev Biol. 2017. PMID: 28207193 Free PMC article. Review.
References
-
- Niederreither K., Vermot J., Messaddeq N., Schuhbaur B., Chambon P., Dollé P. (2001) Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128, 1019–1031 - PubMed
-
- Chen F., Desai T. J., Qian J., Niederreither K., Lü J., Cardoso W. V. (2007) Inhibition of Tgfβ signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 134, 2969–2979 - PubMed
-
- Esteban-Pretel G., Marín M. P., Renau-Piqueras J., Barber T., Timoneda J. (2010) Vitamin A deficiency alters rat lung alveolar basement membrane: reversibility by retinoic acid. J. Nutr. Biochem. 21, 227–236 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

