Glycoepitopes of staphylococcal wall teichoic acid govern complement-mediated opsonophagocytosis via human serum antibody and mannose-binding lectin
- PMID: 24045948
- PMCID: PMC3829409
- DOI: 10.1074/jbc.M113.509893
Glycoepitopes of staphylococcal wall teichoic acid govern complement-mediated opsonophagocytosis via human serum antibody and mannose-binding lectin
Abstract
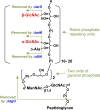
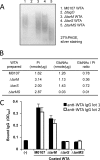
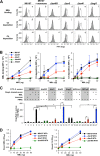
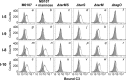
Serum antibodies and mannose-binding lectin (MBL) are important host defense factors for host adaptive and innate immunity, respectively. Antibodies and MBL also initiate the classical and lectin complement pathways, respectively, leading to opsonophagocytosis. We have shown previously that Staphylococcus aureus wall teichoic acid (WTA), a cell wall glycopolymer consisting of ribitol phosphate substituted with α- or β-O-N-acetyl-d-glucosamine (GlcNAc) and d-alanine, is recognized by MBL and serum anti-WTA IgG. However, the exact antigenic determinants to which anti-WTA antibodies or MBL bind have not been determined. To answer this question, several S. aureus mutants, such as α-GlcNAc glycosyltransferase-deficient S. aureus ΔtarM, β-GlcNAc glycosyltransferase-deficient ΔtarS, and ΔtarMS double mutant cells, were prepared from a laboratory and a community-associated methicillin-resistant S. aureus strain. Here, we describe the unexpected finding that β-GlcNAc WTA-deficient ΔtarS mutant cells (which have intact α-GlcNAc) escape from anti-WTA antibody-mediated opsonophagocytosis, whereas α-GlcNAc WTA-deficient ΔtarM mutant cells (which have intact β-GlcNAc) are efficiently engulfed by human leukocytes via anti-WTA IgG. Likewise, MBL binding in S. aureus cells was lost in the ΔtarMS double mutant but not in either single mutant. When we determined the serum concentrations of the anti-α- or anti-β-GlcNAc-specific WTA IgGs, anti-β-GlcNAc WTA-IgG was dominant in pooled human IgG fractions and in the intact sera of healthy adults and infants. These data demonstrate the importance of the WTA sugar conformation for human innate and adaptive immunity against S. aureus infection.
Keywords: Cell Wall; Complement System; Gram-positive Bacteria; Host Defense; Host-Pathogen Interactions; Innate Immunity; S. aureus.
Figures

References
-
- Lowy F. D. (1998) Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 - PubMed
-
- Weidenmaier C., Peschel A. (2008) Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 6, 276–287 - PubMed
-
- Xia G., Kohler T., Peschel A. (2010) The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int. J. Med. Microbiol. 300, 148–154 - PubMed
-
- Weidenmaier C., Kokai-Kun J. F., Kristian S. A., Chanturiya T., Kalbacher H., Gross M., Nicholson G., Neumeister B., Mond J. J., Peschel A. (2004) Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10, 243–245 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

