Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions
- PMID: 24045991
- PMCID: PMC3805087
- DOI: 10.1167/iovs.13-12551
Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions
Abstract
Purpose: We compared gene expression signatures in tree shrew sclera produced by three different visual conditions that all produce ocular elongation and myopia: minus-lens wear, form deprivation, and dark treatment.
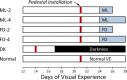
Methods: Six groups of tree shrews (n = 7 per group) were used. Starting 24 days after normal eye-opening (days of visual experience [DVE]), two minus-lens groups wore a monocular -5 diopter (D) lens for 2 days (ML-2) or 4 days (ML-4); two form-deprivation groups wore a monocular translucent diffuser for 2 days (FD-2) or 4 days (FD-4). A dark-treatment (DK) group was placed in continuous darkness for 11 days after experiencing a light/dark environment until 17 DVE. A normal colony-reared group was examined at 28 DVE. Quantitative PCR was used to measure the relative differences in mRNA levels for 55 candidate genes in the sclera that were selected, either because they showed differential expression changes in previous ML studies or because a whole-transcriptome analysis suggested they would change during myopia development.
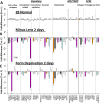
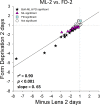
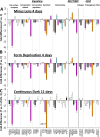
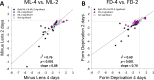
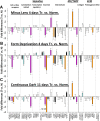
Results: The treated eyes in all groups responded with a significant myopic shift, indicating that the myopia was actively progressing. In the ML-2 group, 27 genes were significantly downregulated in the treated eyes, relative to control eyes. In the treated eyes of the FD-2 group, 16 of the same genes also were significantly downregulated and one was upregulated. The two gene expression patterns were significantly correlated (r(2) = 0.90, P < 0.001). After 4 days of treatment, 31 genes were significantly downregulated in the treated eyes of the ML-4 group and three were upregulated. Twenty-nine of the same genes (26 down- and 3 up-regulated) and six additional genes (all downregulated) were significantly affected in the FD-4 group. The response patterns were highly correlated (r(2) = 0.95, P < 0.001). When the DK group (mean of right and left eyes) was compared to the control eyes of the ML-4 group, the direction and magnitude of the gene expression patterns were similar to those of the ML-4 (r(2) = 0.82, P < 0.001, excluding PENK). Similar patterns also were found when the treated eyes of the ML-4, FD-4, and DK groups were compared to the age-matched normal eyes.
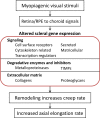
Conclusions: The very similar gene expression signatures produced in the sclera by the three different myopiagenic visual conditions at different time points suggests that there is a "scleral remodeling signature" in this mammal, closely related to primates. The scleral genes examined did not distinguish between the specific visual stimuli that initiate the signaling cascade that results in axial elongation and myopia.
Keywords: animal models; axial elongation; emmetropization; gene expression; myopia; refractive error; sclera.
Figures



References
-
- Sperduto RD, Seigel D, Roberts J, Rowland M. Prevalence of myopia in the United States. Arch Ophthalmol. 1983; 101: 405–407 - PubMed
-
- Fledelius HC. Myopia prevalence in Scandinavia. A survey, with emphasis on factors of relevance for epidemiological refraction studies in general. Acta Ophthalmol Suppl. 1988; 185: 44–50 - PubMed
-
- Wang Q, Klein BE, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1994; 35: 4344–4347 - PubMed
-
- Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology. 1999; 106: 1066–1072 - PubMed
-
- Wensor M, McCarty CA, Taylor HR. Prevalence and risk factors of myopia in Victoria, Australia. Arch Ophthalmol. 1999; 117: 658–663 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

