The developmental origins of adipose tissue
- PMID: 24046315
- PMCID: PMC3775412
- DOI: 10.1242/dev.080549
The developmental origins of adipose tissue
Abstract
Adipose tissue is formed at stereotypic times and locations in a diverse array of organisms. Once formed, the tissue is dynamic, responding to homeostatic and external cues and capable of a 15-fold expansion. The formation and maintenance of adipose tissue is essential to many biological processes and when perturbed leads to significant diseases. Despite this basic and clinical significance, understanding of the developmental biology of adipose tissue has languished. In this Review, we highlight recent efforts to unveil adipose developmental cues, adipose stem cell biology and the regulators of adipose tissue homeostasis and dynamism.
Keywords: Adipocyte; Adipose; Development; Niche; Stem cells; Vascular niche.
Figures
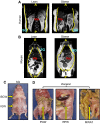
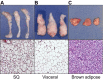
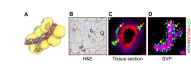
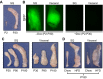

References
-
- Akazawa S., Sun F., Ito M., Kawasaki E., Eguchi K. (2000). Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care 23, 1067–1071 - PubMed
-
- Atit R., Sgaier S. K., Mohamed O. A., Taketo M. M., Dufort D., Joyner A. L., Niswander L., Conlon R. A. (2006). Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 296, 164–176 - PubMed
-
- Barak Y., Nelson M. C., Ong E. S., Jones Y. Z., Ruiz-Lozano P., Chien K. R., Koder A., Evans R. M. (1999). PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

