An insert-based enzymatic cell culture system to rapidly and reversibly induce hypoxia: investigations of hypoxia-induced cell damage, protein expression and phosphorylation in neuronal IMR-32 cells
- PMID: 24046359
- PMCID: PMC3820273
- DOI: 10.1242/dmm.013078
An insert-based enzymatic cell culture system to rapidly and reversibly induce hypoxia: investigations of hypoxia-induced cell damage, protein expression and phosphorylation in neuronal IMR-32 cells
Abstract
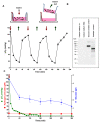
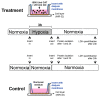
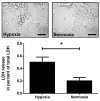
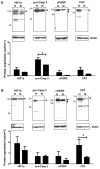
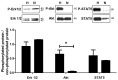
Ischemia-reperfusion injury and tissue hypoxia are of high clinical relevance because they are associated with various pathophysiological conditions such as myocardial infarction and stroke. Nevertheless, the underlying mechanisms causing cell damage are still not fully understood, which is at least partially due to the lack of cell culture systems for the induction of rapid and transient hypoxic conditions. The aim of the study was to establish a model that is suitable for the investigation of cellular and molecular effects associated with transient and long-term hypoxia and to gain insights into hypoxia-mediated mechanisms employing a neuronal culture system. A semipermeable membrane insert system in combination with the hypoxia-inducing enzymes glucose oxidase and catalase was employed to rapidly and reversibly generate hypoxic conditions in the culture medium. Hydrogen peroxide assays, glucose measurements and western blotting were performed to validate the system and to evaluate the effects of the generated hypoxia on neuronal IMR-32 cells. Using the insert-based two-enzyme model, hypoxic conditions were rapidly induced in the culture medium. Glucose concentrations gradually decreased, whereas levels of hydrogen peroxide were not altered. Moreover, a rapid and reversible (on-off) generation of hypoxia could be performed by the addition and subsequent removal of the enzyme-containing inserts. Employing neuronal IMR-32 cells, we showed that 3 hours of hypoxia led to morphological signs of cellular damage and significantly increased levels of lactate dehydrogenase (a biochemical marker of cell damage). Hypoxic conditions also increased the amounts of cellular procaspase-3 and catalase as well as phosphorylation of the pro-survival kinase Akt, but not Erk1/2 or STAT5. In summary, we present a novel framework for investigating hypoxia-mediated mechanisms at the cellular level. We claim that the model, the first of its kind, enables researchers to rapidly and reversibly induce hypoxic conditions in vitro without unwanted interference of the hypoxia-inducing agent on the cultured cells. The system could help to further unravel hypoxia-associated mechanisms that are clinically relevant in various tissues and organs.
Figures

References
-
- Abudara V., Jiang R. G., Eyzaguirre C. (2002). Behavior of junction channels between rat glomus cells during normoxia and hypoxia. J. Neurophysiol. 88, 639–649 - PubMed
-
- Allen C. B., Schneider B. K., White C. W. (2001). Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am. J. Physiol. 281, L1021–L1027 - PubMed
-
- Alqawi O., Wang H. P., Espiritu M., Singh G. (2007). Chronic hypoxia promotes an aggressive phenotype in rat prostate cancer cells. Free Radic. Res. 41, 788–797 - PubMed
-
- Araya R., Uehara T., Nomura Y. (1998). Hypoxia induces apoptosis in human neuroblastoma SK-N-MC cells by caspase activation accompanying cytochrome c release from mitochondria. FEBS Lett. 439, 168–172 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

