Cadmium induced cell apoptosis, DNA damage, decreased DNA repair capacity, and genomic instability during malignant transformation of human bronchial epithelial cells
- PMID: 24046522
- PMCID: PMC3775105
- DOI: 10.7150/ijms.6308
Cadmium induced cell apoptosis, DNA damage, decreased DNA repair capacity, and genomic instability during malignant transformation of human bronchial epithelial cells
Retraction in
-
Retraction: Cadmium induced cell apoptosis, DNA damage, decreased DNA repair capacity, and genomic instability during malignant transformation of human bronchial epithelial cells.Int J Med Sci. 2014 Jan 15;11(3):246. doi: 10.7150/ijms.8533. eCollection 2014. Int J Med Sci. 2014. PMID: 24516347 Free PMC article. No abstract available.
Abstract
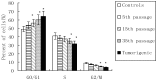
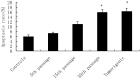
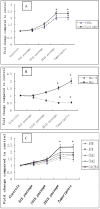
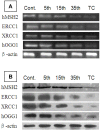
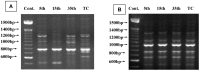
Cadmium and its compounds are well-known human carcinogens, but the mechanisms underlying the carcinogenesis are not entirely understood. Our study was designed to elucidate the mechanisms of DNA damage in cadmium-induced malignant transformation of human bronchial epithelial cells. We analyzed cell cycle, apoptosis, DNA damage, gene expression, genomic instability, and the sequence of exons in DNA repair genes in several kinds of cells. These cells consisted of untreated control cells, cells in the fifth, 15th, and 35th passage of cadmium-treated cells, and tumorigenic cells from nude mice using flow cytometry, Hoechst 33258 staining, comet assay, quantitative real-time polymerase chain reaction (PCR), Western blot analysis, random amplified polymorphic DNA (RAPD)-PCR, and sequence analysis. We observed a progressive increase in cell population of the G0/G1 phase of the cell cycle and the rate of apoptosis, DNA damage, and cadmium-induced apoptotic morphological changes in cerebral cortical neurons during malignant transformation. Gene expression analysis revealed increased expression of cell proliferation (PCNA), cell cycle (CyclinD1), pro-apoptotic activity (Bax), and DNA damage of the checkpoint genes ATM, ATR, Chk1, Chk2, Cdc25A. Decreased expression of the anti-apoptotic gene Bcl-2 and the DNA repair genes hMSH2, hMLH1, ERCC1, ERCC2, and hOGG1 was observed. RAPD-PCR revealed genomic instability in cadmium-exposed cells, and sequence analysis showed mutation of exons in hMSH2, ERCC1, XRCC1, and hOGG1 in tumorigenic cells. This study suggests that Cadmium can increase cell apoptosis and DNA damage, decrease DNA repair capacity, and cause mutations, and genomic instability leading to malignant transformation. This process could be a viable mechanism for cadmium-induced cancers.
Keywords: Cadmium chloride; DNA damage; DNA repair genes; genomic instability..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Waalkes MP. Cadmium carcinogenesis. Mutat Res. 2003;533:107–120. - PubMed
-
- Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PEB, Williams DJ, Moore MR. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett. 2003;137:65–83. - PubMed
-
- Swaddiwudhipong W, Mahasakpan P, Funkhiew T, Limpatanachote P. Changes in cadmium exposure among persons living in cadmium-contaminated areas in northwestern Thailand: a five-year follow-up. J Med Assoc Thai. 2010;93:1217–1222. - PubMed
-
- Koyu A, Gokcimen A, Ozguner F, Bayram DS, Kocak A. Evaluation of the effects of cadmium on rat liver. Mol Cell Biochem. 2006;20:1–5. - PubMed
-
- Nordberg G, Jin T, Bernard A, Fierens S, Buchet JP, Ye T, Kong Q, Wang H. Low bone density and renal dysfunction following environmental cadmium exposure in China. Ambio. 2002;31:478–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

