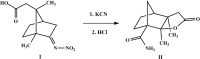2,6-Dimethyl-4-oxo-3-oxatri-cyclo-[5.2.1.0(2,6)]decane-1-carboxamide
- PMID: 24046712
- PMCID: PMC3770427
- DOI: 10.1107/S1600536813017248
2,6-Dimethyl-4-oxo-3-oxatri-cyclo-[5.2.1.0(2,6)]decane-1-carboxamide
Abstract
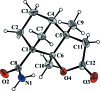
In the title compound, C12H17NO3, which was synthesized by Wagner-Meerwein rearrangement of the N-nitro-imine, the ring-junction C-C bond length is comparatively long [1.573 (2) Å] due to a steric repulsion between the methyl groups at these atoms, which also leads to an increase in the C-C-C angles along this C4 chain [118.10 (13) and 115.04 (15) °, respectively]. In the crystal, N-H⋯O-C and N-H⋯O=C hydrogen bonds are formed between the amide group and the two O-atom acceptors of the lactone group, forming a chain along [001].
Figures
References
-
- Bondavalli, F., Bruno, O., Ranise, A., Schenone, P., Susanna, V., Lisa, M., Maione, S. & Marmo, E. (1987). Il Farmaco, 42, 947–953. - PubMed
-
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2008). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bulman Page, P. C., Murrell, V. L., Limousin, C., Laffan, D. D. P., Bethell, D., Slawin, A. M. Z. & Smith, T. A. D. (2000). J. Org. Chem. 65, 4204–4207. - PubMed
-
- Knollmuller, M., Gartner, P., Ferencic, M., Noe, C. R. & Mereiter, K. (1998). Eur. J. Org. Chem. pp. 2507–2511.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous