Therapeutic strategies impacting cancer cell glutamine metabolism
- PMID: 24047273
- PMCID: PMC4154374
- DOI: 10.4155/fmc.13.130
Therapeutic strategies impacting cancer cell glutamine metabolism
Abstract
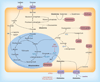
The metabolic adaptations that support oncogenic growth can also render cancer cells dependent on certain nutrients. Along with the Warburg effect, increased utilization of glutamine is one of the metabolic hallmarks of the transformed state. Glutamine catabolism is positively regulated by multiple oncogenic signals, including those transmitted by the Rho family of GTPases and by c-Myc. The recent identification of mechanistically distinct inhibitors of glutaminase, which can selectively block cellular transformation, has revived interest in the possibility of targeting glutamine metabolism in cancer therapy. Here, we outline the regulation and roles of glutamine metabolism within cancer cells and discuss possible strategies for, and the consequences of, impacting these processes therapeutically.
Figures

References
-
- Warburg O. Origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
-
- Warburg O. Respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. - PubMed
-
- Warburg O, Posener K, Negelein E. On the metabolism of carcinoma cells. Biochemische Zeitschrift. 1924;152:309–344.
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11(2):85–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
