Direct transdifferentiation of spermatogonial stem cells to morphological, phenotypic and functional hepatocyte-like cells via the ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin E
- PMID: 24047406
- PMCID: PMC3848919
- DOI: 10.1186/1478-811X-11-67
Direct transdifferentiation of spermatogonial stem cells to morphological, phenotypic and functional hepatocyte-like cells via the ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin E
Abstract
Background: Severe shortage of liver donors and hepatocytes highlights urgent requirement of extra-liver and stem cell source of hepatocytes for treating liver-related diseases. Here we hypothesized that spermatogonial stem cells (SSCs) can directly transdifferentiate to hepatic stem-like cells capable of differentiating into mature hepatocyte-like cells in vitro without an intervening pluripotent state.
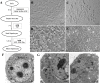
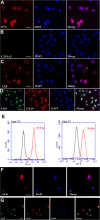
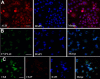
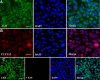
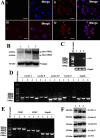
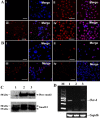
Results: SSCs first changed into hepatic stem-like cells since they resembled hepatic oval cells in morphology and expressed Ck8, Ck18, Ck7, Ck19, OV6, and albumin. Importantly, they co-expressed CK8 and CK19 but not ES cell markers. Hepatic stem-like cells derived from SSCs could differentiate into small hepatocytes based upon their morphological features and expression of numerous hepatic cell markers but lacking of bile epithelial cell hallmarks. Small hepatocytes were further coaxed to differentiate into mature hepatocyte-like cells, as identified by their morphological traits and strong expression of Ck8, Ck18, Cyp7a1, Hnf3b, Alb, Tat, Ttr, albumin, and CYP1A2 but not Ck7 or CK19. Notably, these differentiated cells acquired functional attributes of hepatocyte-like cells because they secreted albumin, synthesized urea, and uptake and released indocyanine green. Moreover, phosphorylation of ERK1/2 and Smad2/3 rather than Akt was activated in hepatic stem cells and mature hepatocytes. Additionally, cyclin A, cyclin B and cyclin E transcripts and proteins but not cyclin D1 or CDK1 and CDK2 transcripts or proteins were reduced in mature hepatocyte-like cells or hepatic stem-like cells derived from SSCs compared to SSCs.
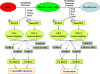
Conclusions: SSCs can transdifferentiate to hepatic stem-like cells capable of differentiating into cells with morphological, phenotypic and functional characteristics of mature hepatocytes via the activation of ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin E. This study thus provides an invaluable source of mature hepatocytes for treating liver-related diseases and drug toxicity screening and offers novel insights into mechanisms of liver development and cell reprogramming.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

