S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis
- PMID: 24047412
- PMCID: PMC3863739
- DOI: 10.1164/rccm.201304-0803OC
S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis
Abstract
Rationale: A hallmark of pulmonary tuberculosis (TB) is the formation of granulomas. However, the immune factors that drive the formation of a protective granuloma during latent TB, and the factors that drive the formation of inflammatory granulomas during active TB, are not well defined.
Objectives: The objective of this study was to identify the underlying immune mechanisms involved in formation of inflammatory granulomas seen during active TB.
Methods: The immune mediators involved in inflammatory granuloma formation during TB were assessed using human samples and experimental models of Mycobacterium tuberculosis infection, using molecular and immunologic techniques.
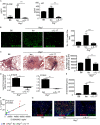
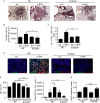
Measurements and main results: We demonstrate that in human patients with active TB and in nonhuman primate models of M. tuberculosis infection, neutrophils producing S100 proteins are dominant within the inflammatory lung granulomas seen during active TB. Using the mouse model of TB, we demonstrate that the exacerbated lung inflammation seen as a result of neutrophilic accumulation is dependent on S100A8/A9 proteins. S100A8/A9 proteins promote neutrophil accumulation by inducing production of proinflammatory chemokines and cytokines, and influencing leukocyte trafficking. Importantly, serum levels of S100A8/A9 proteins along with neutrophil-associated chemokines, such as keratinocyte chemoattractant, can be used as potential surrogate biomarkers to assess lung inflammation and disease severity in human TB.
Conclusions: Our results thus show a major pathologic role for S100A8/A9 proteins in mediating neutrophil accumulation and inflammation associated with TB. Thus, targeting specific molecules, such as S100A8/A9 proteins, has the potential to decrease lung tissue damage without impacting protective immunity against TB.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL079142/HL/NHLBI NIH HHS/United States
- AI91036/AI/NIAID NIH HHS/United States
- R56 AI058131/AI/NIAID NIH HHS/United States
- R01 AI076389/AI/NIAID NIH HHS/United States
- R01 AI060422/AI/NIAID NIH HHS/United States
- HL105427/HL/NHLBI NIH HHS/United States
- P51 RR000164/RR/NCRR NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- AI091457/AI/NIAID NIH HHS/United States
- R01 HL105427/HL/NHLBI NIH HHS/United States
- RR020159/RR/NCRR NIH HHS/United States
- AI060422/AI/NIAID NIH HHS/United States
- AI058131/AI/NIAID NIH HHS/United States
- RR000164/RR/NCRR NIH HHS/United States
- P20 RR020159/RR/NCRR NIH HHS/United States
- AI076389/AI/NIAID NIH HHS/United States
- R01 AI058131/AI/NIAID NIH HHS/United States
- R21 AI091457/AI/NIAID NIH HHS/United States
- RR026006/RR/NCRR NIH HHS/United States
- T32 AI065380-08/AI/NIAID NIH HHS/United States
- U19 AI091036/AI/NIAID NIH HHS/United States
- R21 RR026006/RR/NCRR NIH HHS/United States
- T32 AI065380/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

